当前位置:
X-MOL 学术
›
Atmos. Environ.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Atmospheric SO2 oxidation by NO2 plays no role in the mass independent sulfur isotope fractionation of urban aerosols
Atmospheric Environment ( IF 4.2 ) Pub Date : 2018-11-01 , DOI: 10.1016/j.atmosenv.2018.09.007 D. Au Yang , G. Bardoux , N. Assayag , C. Laskar , D. Widory , P. Cartigny
Atmospheric Environment ( IF 4.2 ) Pub Date : 2018-11-01 , DOI: 10.1016/j.atmosenv.2018.09.007 D. Au Yang , G. Bardoux , N. Assayag , C. Laskar , D. Widory , P. Cartigny
|
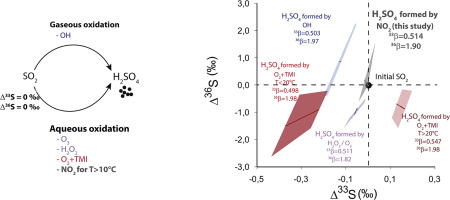
Abstract Modern anthropogenic aerosols usually exhibit low but significant Δ33S signatures (−0.6 to 0.5‰) whose origin still remains unclear. While isotope fractionation factors associated with the oxidation of SO2 by O2+TMI (Transition Metal Ion), H2O2 or OH cannot lead to such extreme Δ33S-values, an increasing number of studies points to the significant role of NO2 as a contributing oxidant, especially in the urban environment. To address the possible relation between atmospheric NO2 and observed Δ33S-values in aerosols, we carried out laboratory experiments oxidizing SO2 by NO2 at temperatures ranging between −7 and 52 °C. Our results show that at temperatures ≥10 °C SO2 oxidation by NO2 is characterized by 1) a 34α-value whose temperature dependence (0.2437/T+0.0457) is distinct from those related to oxidation by O2+TMI, H2O2 and OH oxidation pathways and 2) 33β (0.514 ± 0.0003) and 36β (1.90 ± 0.002) values that are closer to the mass dependent values (0.515 and 1.89 respectively) than those reported for the other oxidation pathways. This implies that the NO2 oxidation pathway cannot explain the extreme Δ33S-values measured in urban aerosols. Our data show that if atmospheric SO2 oxidation by NO2 is neglected, both the O2+TMI and OH oxidation pathways would be overestimated in urban areas. Finally, we conclude that another oxidation reaction is responsible for the high Δ33S-values measured in urban aerosol samples.
中文翻译:

NO2 对大气 SO2 的氧化在城市气溶胶的与质量无关的硫同位素分馏中没有作用
摘要 现代人为气溶胶通常表现出低但显着的 Δ33S 特征(-0.6 至 0.5‰),其来源仍不清楚。虽然与 O2+TMI(过渡金属离子)、H2O2 或 OH 氧化 SO2 相关的同位素分馏因素不能导致如此极端的 Δ33S 值,但越来越多的研究指出 NO2 作为助氧化剂的重要作用,尤其是在城市环境中。为了解决大气 NO2 与观测到的气溶胶 Δ33S 值之间可能存在的关系,我们进行了实验室实验,在 -7 到 52 °C 的温度范围内用 NO2 氧化 SO2。我们的结果表明,在 10 °C 以上的温度下,NO2 氧化 SO2 的特征在于 1) 34α 值,其温度依赖性 (0.2437/T+0.0457) 与 O2+TMI 氧化相关的那些不同,H2O2 和 OH 氧化途径和 2) 33β (0.514 ± 0.0003) 和 36β (1.90 ± 0.002) 值比其他氧化途径报告的值更接近质量依赖值(分别为 0.515 和 1.89)。这意味着 NO2 氧化途径无法解释在城市气溶胶中测量的极端 Δ33S 值。我们的数据表明,如果忽略 NO2 对大气 SO2 的氧化,城市地区的 O2+TMI 和 OH 氧化途径都会被高估。最后,我们得出结论,另一种氧化反应是造成城市气溶胶样品中测量的高 Δ33S 值的原因。这意味着 NO2 氧化途径无法解释在城市气溶胶中测量的极端 Δ33S 值。我们的数据表明,如果忽略 NO2 对大气 SO2 的氧化,城市地区的 O2+TMI 和 OH 氧化途径都会被高估。最后,我们得出结论,另一种氧化反应是造成城市气溶胶样品中测量的高 Δ33S 值的原因。这意味着 NO2 氧化途径无法解释在城市气溶胶中测量的极端 Δ33S 值。我们的数据表明,如果忽略 NO2 对大气 SO2 的氧化,城市地区的 O2+TMI 和 OH 氧化途径都会被高估。最后,我们得出结论,另一种氧化反应是造成城市气溶胶样品中测量的高 Δ33S 值的原因。
更新日期:2018-11-01
中文翻译:

NO2 对大气 SO2 的氧化在城市气溶胶的与质量无关的硫同位素分馏中没有作用
摘要 现代人为气溶胶通常表现出低但显着的 Δ33S 特征(-0.6 至 0.5‰),其来源仍不清楚。虽然与 O2+TMI(过渡金属离子)、H2O2 或 OH 氧化 SO2 相关的同位素分馏因素不能导致如此极端的 Δ33S 值,但越来越多的研究指出 NO2 作为助氧化剂的重要作用,尤其是在城市环境中。为了解决大气 NO2 与观测到的气溶胶 Δ33S 值之间可能存在的关系,我们进行了实验室实验,在 -7 到 52 °C 的温度范围内用 NO2 氧化 SO2。我们的结果表明,在 10 °C 以上的温度下,NO2 氧化 SO2 的特征在于 1) 34α 值,其温度依赖性 (0.2437/T+0.0457) 与 O2+TMI 氧化相关的那些不同,H2O2 和 OH 氧化途径和 2) 33β (0.514 ± 0.0003) 和 36β (1.90 ± 0.002) 值比其他氧化途径报告的值更接近质量依赖值(分别为 0.515 和 1.89)。这意味着 NO2 氧化途径无法解释在城市气溶胶中测量的极端 Δ33S 值。我们的数据表明,如果忽略 NO2 对大气 SO2 的氧化,城市地区的 O2+TMI 和 OH 氧化途径都会被高估。最后,我们得出结论,另一种氧化反应是造成城市气溶胶样品中测量的高 Δ33S 值的原因。这意味着 NO2 氧化途径无法解释在城市气溶胶中测量的极端 Δ33S 值。我们的数据表明,如果忽略 NO2 对大气 SO2 的氧化,城市地区的 O2+TMI 和 OH 氧化途径都会被高估。最后,我们得出结论,另一种氧化反应是造成城市气溶胶样品中测量的高 Δ33S 值的原因。这意味着 NO2 氧化途径无法解释在城市气溶胶中测量的极端 Δ33S 值。我们的数据表明,如果忽略 NO2 对大气 SO2 的氧化,城市地区的 O2+TMI 和 OH 氧化途径都会被高估。最后,我们得出结论,另一种氧化反应是造成城市气溶胶样品中测量的高 Δ33S 值的原因。











































 京公网安备 11010802027423号
京公网安备 11010802027423号