Ultrasonics Sonochemistry ( IF 8.7 ) Pub Date : 2018-09-05 , DOI: 10.1016/j.ultsonch.2018.08.028 Takshak Shende , Gangadhar Andaluri , Rominder P.S. Suri
|
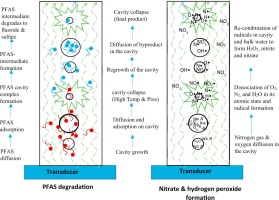
Sonolytic degradation kinetics of non-volatile surfactant perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) were investigated over a range of concentration, considering active cavity as a catalyst. The Michaelis-Menten type kinetic model was developed to empirically estimate the concentration of active cavity sites during reactions. Sonolytic degradation of PFOA and PFOS, as well as the formation of its inorganic constituents, fluoride, and sulfate, follows saturation kinetics of pseudo-first order at lower concentration (<2.34 µM) and zero order at higher concentration (>23.60 µM). Nitrate and hydrogen peroxide formations were 0.53 ± 0.14 µM/min and 0.95 ± 0.11 µM/min, respectively. At a power density of 77 W/L and frequency of 575 kHz, the empirically estimated maximum number of active cavity sites that could lead to the sonolytic reaction were 89.25 and 8.8 mM for PFOA and PFOS, respectively. This study suggests that a lower number of active cavity sites with higher temperature needed to degrade PFOS might be the reason for lower degradation rate of PFOS compared to that of PFOA. Diffusion of non-volatile surfactants at the cavity-water interface is found to be the rate-limiting step for the mineralization of perfluoroalkyl substances.
中文翻译:

非挥发性表面活性剂声降解的动力学模型:全氟烷基物质
以活性腔为催化剂,研究了非挥发性表面活性剂全氟辛酸(PFOA)和全氟辛烷磺酸(PFOS)的声降解动力学。开发了Michaelis-Menten型动力学模型以凭经验估算反应过程中活性腔位的浓度。PFOA和PFOS的声分解降解,以及其无机成分,氟化物和硫酸盐的形成,在较低浓度(<2.34 µM)时为拟一级反应,在较高浓度(> 23.60 µM)时为零级反应。硝酸盐和过氧化氢的形成分别为0.53±0.14 µM / min和0.95±0.11 µM / min。在77 W / L的功率密度和575 kHz的频率下,根据经验估计,PFOA和PFOS可能导致声波反应的最大空腔位置分别为89.25和8.8 mM。这项研究表明,与全氟辛烷磺酸相比,降解全氟辛烷磺酸所需的具有较高温度的活性腔位点数量较少可能是导致全氟辛烷磺酸降解率较低的原因。发现非挥发性表面活性剂在型腔-水界面的扩散是全氟烷基物质矿化的限速步骤。











































 京公网安备 11010802027423号
京公网安备 11010802027423号