Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2018-09-05 , DOI: 10.1016/j.bmcl.2018.09.004 Guangcheng Wang , Zhiyun Peng , Shanshan Peng , Jie Qiu , Yongjun Li , Yanyu Lan
|
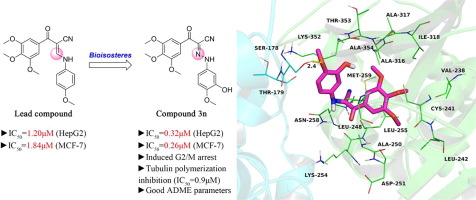
A series of (E)-N-Aryl-2-oxo-2-(3,4,5-trimethoxyphenyl)acetohydrazonoyl cyanides have been synthesized and evaluated for their anticancer activity in human hepatocellular liver carcinoma HepG2 and breast adenocarcinoma MCF-7 cell lines. Among all the tested compounds, compound 3a, 3e and 3n displayed more activity than lead compound with IC50 value of 0.26–0.61 μM. Meanwhile, these compounds (3a, 3e and 3n) showed potent antiproliferative activity against a panel of cancer cells and the HCT-8/T multidrug resistant cell line with IC50 values in the range of 0.077– 7.44 μM. Flow cytometric analyses revealed that compound 3n induced cell cycle arrest in G2/M phases in a dose dependent manner. The compound 3n also displayed potent tubulin polymerization inhibition with an IC50 value of 0.9 µM, with ten folds more active than colchicine (IC50 = 9 μM). Molecular docking studies revealed that compound 3n efficiently interacted with the colchicine binding site of tubulin through hydrophobic, cation-π and hydrogen bond interaction. Furthermore, in silico pharmacokinetic prediction shown that these compounds have a good ADME-related physicochemical parameters. These results demonstrate that 3n exhibits potent cytotoxicity in cancer cells by targeting the colchicine binding site of tubulin and potentially acts as a therapeutic lead compound for the development of anticancer drugs.
中文翻译:

(E)-N-芳基-2-氧代-2-(3,4,5-三甲氧基苯基)乙酰肼基酰基氰作为微管蛋白聚合抑制剂:基于结构的生物等排体设计,合成,生物学评估,分子对接和计算机模拟ADME预测
合成了一系列(E)-N-Aryl-2-oxo-2-(3,4,5-三甲氧基苯基)乙酰肼基酰氰化物,并评估了其在人肝细胞肝癌HepG2和乳腺癌中的抗癌活性MCF-7细胞线。在所有测试化合物中,化合物3a,3e和3n的活性均高于铅化合物,IC 50值为0.26-0.61μM。同时,这些化合物(3a,3e和3n)对一组癌细胞和HCT-8 / T多药耐药细胞系显示有效的抗增殖活性,IC 50值在0.077至7.44μM之间。流式细胞仪分析表明该化合物3n诱导的细胞周期阻滞于G2 / M期,呈剂量依赖性。化合物3n还显示出有效的微管蛋白聚合抑制作用,IC 50值为0.9 µM,活性比秋水仙碱高十倍(IC 50 = 9 µM)。分子对接研究表明,化合物3n通过疏水,阳离子-π和氢键相互作用与微管蛋白的秋水仙碱结合位点有效相互作用。此外,计算机计算机动力学预测表明,这些化合物具有良好的ADME相关的理化参数。这些结果表明3n 通过靶向微管蛋白的秋水仙碱结合位点,在癌细胞中具有强的细胞毒性,并有可能作为开发抗癌药物的治疗先导化合物。









































 京公网安备 11010802027423号
京公网安备 11010802027423号