当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dissecting the Mechanism of Oligomerization and Macrocyclization Reactions of NRPS‐Independent Siderophore Synthetases
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2018-10-17 , DOI: 10.1002/chem.201803494 Sina Rütschlin 1 , Thomas Böttcher 1
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2018-10-17 , DOI: 10.1002/chem.201803494 Sina Rütschlin 1 , Thomas Böttcher 1
Affiliation
|
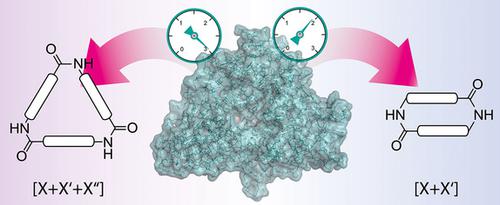
Macrocyclic and linear hydroxamate siderophores produced by NRPS‐independent siderophore (NIS; NRPS=nonribosomal peptide synthetase) synthetases are important in the bacterial competition for iron, as virulence factors, and as drugs for medical use in humans. Despite their importance, the mechanistic details of NIS synthetases have so far remained obscure. Using synthetic substrate analogues as tools allowed for an interrogation of the mechanism of the two closely related NIS synthetases AvbD and DesD. While AvbD produces macrocyclic homo‐ and heterodimers as native products, DesD is responsible for the synthesis of trimeric desferrioxamines. These enzymes comprise two adjacent binding sites with different substrate selectivities, which direct oligomerization and macrocyclization steps. Exploiting this difference, synthetic substrates were used to invert the native affinities for the sites resulting in switching from trimerization to dimerization reactions for DesD. Based on this work, a comprehensive model explaining the mechanistic details of the reactions and the differences between trimerizing and dimerizing enzymes was developed. Finally, a DesD mutant demonstrated the tuneability of the enzyme's substrate selectivity by only minor changes in the protein sequence. This finding confirms the affinity‐directed mechanism responsible for the iterativity of oligomerization and macrocyclization steps.
中文翻译:

解析独立于NRPS的铁载体合成的低聚和大环化反应机理
由NRPS独立的铁载体(NIS; NRPS =非核糖体肽合成酶)合成产生的大环和线性异羟肟酸酯铁载体在细菌竞争铁,作为毒力因子和作为人类医学药物方面很重要。尽管具有重要意义,但NIS合成酶的机理细节至今仍不清楚。使用合成底物类似物作为工具可以询问两个紧密相关的NIS合成酶AvbD和DesD的机理。AvbD生产天然产物大环同二聚体和异二聚体,而DesD负责三聚体去铁胺的合成。这些酶包含具有不同底物选择性的两个相邻结合位点,其指导寡聚和大环化步骤。利用这种差异,合成底物用于反转位点的天然亲和力,从而导致DesD从三聚反应切换为二聚反应。在这项工作的基础上,建立了一个解释反应机理以及三聚酶和二聚酶之间差异的综合模型。最后,DesD突变体仅通过蛋白质序列的微小变化就证明了酶底物选择性的可调性。这一发现证实了导致寡聚化和大环化步骤重复性的亲和力导向机制。建立了一个全面的模型,解释了反应的机理细节以及三聚酶和二聚酶之间的差异。最后,DesD突变体仅通过蛋白质序列的微小变化就证明了酶底物选择性的可调性。这一发现证实了导致寡聚化和大环化步骤重复性的亲和力导向机制。建立了一个全面的模型,解释了反应的机理细节以及三聚酶和二聚酶之间的差异。最后,DesD突变体仅通过蛋白质序列的微小变化就证明了酶底物选择性的可调性。这一发现证实了导致寡聚化和大环化步骤重复性的亲和力导向机制。
更新日期:2018-10-17
中文翻译:

解析独立于NRPS的铁载体合成的低聚和大环化反应机理
由NRPS独立的铁载体(NIS; NRPS =非核糖体肽合成酶)合成产生的大环和线性异羟肟酸酯铁载体在细菌竞争铁,作为毒力因子和作为人类医学药物方面很重要。尽管具有重要意义,但NIS合成酶的机理细节至今仍不清楚。使用合成底物类似物作为工具可以询问两个紧密相关的NIS合成酶AvbD和DesD的机理。AvbD生产天然产物大环同二聚体和异二聚体,而DesD负责三聚体去铁胺的合成。这些酶包含具有不同底物选择性的两个相邻结合位点,其指导寡聚和大环化步骤。利用这种差异,合成底物用于反转位点的天然亲和力,从而导致DesD从三聚反应切换为二聚反应。在这项工作的基础上,建立了一个解释反应机理以及三聚酶和二聚酶之间差异的综合模型。最后,DesD突变体仅通过蛋白质序列的微小变化就证明了酶底物选择性的可调性。这一发现证实了导致寡聚化和大环化步骤重复性的亲和力导向机制。建立了一个全面的模型,解释了反应的机理细节以及三聚酶和二聚酶之间的差异。最后,DesD突变体仅通过蛋白质序列的微小变化就证明了酶底物选择性的可调性。这一发现证实了导致寡聚化和大环化步骤重复性的亲和力导向机制。建立了一个全面的模型,解释了反应的机理细节以及三聚酶和二聚酶之间的差异。最后,DesD突变体仅通过蛋白质序列的微小变化就证明了酶底物选择性的可调性。这一发现证实了导致寡聚化和大环化步骤重复性的亲和力导向机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号