当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Local and Global Dynamics in Intrinsically Disordered Synuclein
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-10-18 , DOI: 10.1002/anie.201808172 Nasrollah Rezaei‐Ghaleh 1 , Giacomo Parigi 2 , Andrea Soranno 3, 4 , Andrea Holla 4 , Stefan Becker 5 , Benjamin Schuler 4 , Claudio Luchinat 2 , Markus Zweckstetter 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-10-18 , DOI: 10.1002/anie.201808172 Nasrollah Rezaei‐Ghaleh 1 , Giacomo Parigi 2 , Andrea Soranno 3, 4 , Andrea Holla 4 , Stefan Becker 5 , Benjamin Schuler 4 , Claudio Luchinat 2 , Markus Zweckstetter 1
Affiliation
|
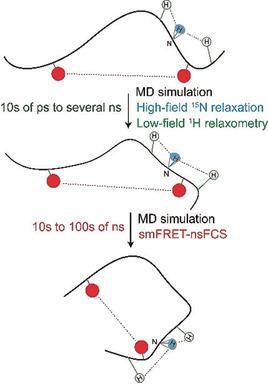
Intrinsically disordered proteins (IDPs) experience a diverse spectrum of motions that are difficult to characterize with a single experimental technique. Herein we combine high‐ and low‐field nuclear spin relaxation, nanosecond fluorescence correlation spectroscopy (nsFCS), and long molecular dynamics simulations of alpha‐synuclein, an IDP involved in Parkinson disease, to obtain a comprehensive picture of its conformational dynamics. The combined analysis shows that fast motions below 2 ns caused by local dihedral angle fluctuations and conformational sampling within and between Ramachandran substates decorrelate most of the backbone N−H orientational memory. However, slow motions with correlation times of up to ca. 13 ns from segmental dynamics are present throughout the alpha‐synuclein chain, in particular in its C‐terminal domain, and global chain reconfiguration occurs on a timescale of ca. 60 ns. Our study demonstrates a powerful strategy to determine residue‐specific protein dynamics in IDPs at different time and length scales.
中文翻译:

固有紊乱突触核蛋白的局部和全局动力学
本质上无序的蛋白质(IDP)经历着各种各样的运动,而这些运动很难用一种实验技术来表征。在这里,我们结合了高场和低场核自旋弛豫,纳秒荧光相关光谱(nsFCS)以及参与帕金森氏病的IDPα-突触核蛋白的长分子动力学模拟,以全面了解其构象动力学。组合分析表明,由局部二面角波动和Ramachandran子状态之内和之间的构象采样引起的2 ns以下的快速运动使大部分骨架NH定向记忆解相关。但是,慢动作的相关时间最多约为。在整个α-突触核蛋白链中,特别是在其C端域中,存在来自段动力学的13 ns,全局链重新配置的时间范围约为。60 ns。我们的研究表明,在不同的时间和长度范围内,确定IDP中残基特异性蛋白质动力学的有效策略。
更新日期:2018-10-18
中文翻译:

固有紊乱突触核蛋白的局部和全局动力学
本质上无序的蛋白质(IDP)经历着各种各样的运动,而这些运动很难用一种实验技术来表征。在这里,我们结合了高场和低场核自旋弛豫,纳秒荧光相关光谱(nsFCS)以及参与帕金森氏病的IDPα-突触核蛋白的长分子动力学模拟,以全面了解其构象动力学。组合分析表明,由局部二面角波动和Ramachandran子状态之内和之间的构象采样引起的2 ns以下的快速运动使大部分骨架NH定向记忆解相关。但是,慢动作的相关时间最多约为。在整个α-突触核蛋白链中,特别是在其C端域中,存在来自段动力学的13 ns,全局链重新配置的时间范围约为。60 ns。我们的研究表明,在不同的时间和长度范围内,确定IDP中残基特异性蛋白质动力学的有效策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号