当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydrogen Atom Transfer Reactions of Mononuclear Nonheme Metal–Oxygen Intermediates
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2018-09-04 00:00:00 , DOI: 10.1021/acs.accounts.8b00299 Wonwoo Nam 1, 2 , Yong-Min Lee 1 , Shunichi Fukuzumi 1, 3
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2018-09-04 00:00:00 , DOI: 10.1021/acs.accounts.8b00299 Wonwoo Nam 1, 2 , Yong-Min Lee 1 , Shunichi Fukuzumi 1, 3
Affiliation
|
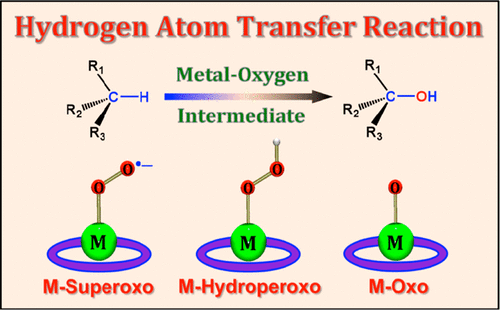
Molecular oxygen (O2), the greenest oxidant, is kinetically stable in the oxidation of organic substrates due to its triplet ground state. In nature, O2 is reduced by two electrons with two protons to produce hydrogen peroxide (H2O2) and by four electrons with four protons to produce water (H2O) by oxidase and oxygenase metalloenzymes. In the process of the two-electron/two-proton and four-electron/four-proton reduction of O2 by metalloenzymes and their model compounds, metal–oxygen intermediates, such as metal–superoxido, −peroxido, −hydroperoxido, and −oxido species, are generated depending on the numbers of electrons and protons involved in the O2 activation reactions. The one-electron reduction of metal–oxygen intermediates is coupled with the binding of one proton. Such a hydrogen atom transfer (HAT) is defined as proton-coupled electron transfer (PCET), and there is a mechanistic dichotomy whether HAT occurs via a concerted PCET pathway or stepwise pathways [i.e., electron transfer followed by proton transfer (ET/PT) or proton transfer followed by electron transfer (PT/ET)]. The metal–oxygen intermediates formed are oxidants that can abstract a hydrogen atom (H-atom) from substrate C–H bonds. The H-atom abstraction from substrate C–H bonds by the metal–oxygen intermediates can also occur via a concerted PCET or stepwise PCET pathways. In the PCET reactions, a proton can be provided not only by the substrate itself but also by an acid that is added to a reaction solution.
中文翻译:

单核非血红素-金属氧中间体的氢原子转移反应
分子氧(O 2)是最绿色的氧化剂,由于其三重态为基态,因此在有机底物的氧化过程中具有动力学稳定性。实际上,O 2通过两个质子被两个电子还原以生成过氧化氢(H 2 O 2),并通过四个质子被四个电子还原以通过氧化酶和加氧酶金属酶生成水(H 2 O)。在金属酶及其模型化合物对O 2的二电子/二质子和四电子/四质子还原过程中,金属-氧中间体,例如金属-过氧化物,-过氧化物,-氢过氧化物和-取决于O 2中所涉及的电子和质子的数量,产生氧化性物质活化反应。金属-氧中间体的单电子还原与一个质子的结合有关。这种氢原子转移(HAT)定义为质子耦合电子转移(PCET),无论是通过一致的PCET途径还是逐步发生的途径[即,电子转移后继质子转移(ET / PT), )或质子转移,然后再进行电子转移(PT / ET)]。形成的金属-氧中间体是氧化剂,可以从底物C-H键中提取氢原子(H原子)。金属-氧中间物从底物C–H键中夺取H原子的过程也可以通过协调一致的PCET或逐步PCET途径发生。在PCET反应中,质子不仅可以由底物本身提供,还可以由添加到反应溶液中的酸提供。
更新日期:2018-09-04
中文翻译:

单核非血红素-金属氧中间体的氢原子转移反应
分子氧(O 2)是最绿色的氧化剂,由于其三重态为基态,因此在有机底物的氧化过程中具有动力学稳定性。实际上,O 2通过两个质子被两个电子还原以生成过氧化氢(H 2 O 2),并通过四个质子被四个电子还原以通过氧化酶和加氧酶金属酶生成水(H 2 O)。在金属酶及其模型化合物对O 2的二电子/二质子和四电子/四质子还原过程中,金属-氧中间体,例如金属-过氧化物,-过氧化物,-氢过氧化物和-取决于O 2中所涉及的电子和质子的数量,产生氧化性物质活化反应。金属-氧中间体的单电子还原与一个质子的结合有关。这种氢原子转移(HAT)定义为质子耦合电子转移(PCET),无论是通过一致的PCET途径还是逐步发生的途径[即,电子转移后继质子转移(ET / PT), )或质子转移,然后再进行电子转移(PT / ET)]。形成的金属-氧中间体是氧化剂,可以从底物C-H键中提取氢原子(H原子)。金属-氧中间物从底物C–H键中夺取H原子的过程也可以通过协调一致的PCET或逐步PCET途径发生。在PCET反应中,质子不仅可以由底物本身提供,还可以由添加到反应溶液中的酸提供。









































 京公网安备 11010802027423号
京公网安备 11010802027423号