当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Molecular Basis for Olefin Rearrangement in the Gephyronic Acid Polyketide Synthase.
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2018-09-12 , DOI: 10.1021/acschembio.8b00645 Greg J Dodge 1 , Danialle Ronnow 2 , Richard E Taylor 2 , Janet L Smith 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2018-09-12 , DOI: 10.1021/acschembio.8b00645 Greg J Dodge 1 , Danialle Ronnow 2 , Richard E Taylor 2 , Janet L Smith 1
Affiliation
|
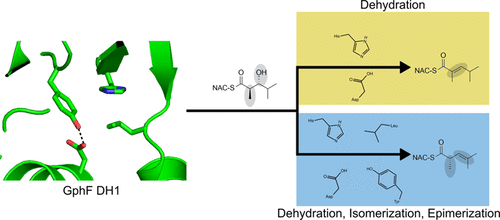
Polyketide synthases (PKS) are a rich source of natural products of varied chemical composition and biological significance. Here, we report the characterization of an atypical dehydratase (DH) domain from the PKS pathway for gephyronic acid, an inhibitor of eukaryotic protein synthesis. Using a library of synthetic substrate mimics, the reaction course, stereospecificity, and tolerance to non-native substrates of GphF DH1 are probed via LC-MS analysis. Taken together, the studies establish GphF DH1 as a dual-function dehydratase/isomerase that installs an odd-to-even double bond and yields a product consistent with the isobutenyl terminus of gephyronic acid. The studies also reveal an unexpected C2 epimerase function in catalytic turnover with the native substrate. A 1.55-Å crystal structure of GphF DH1 guided mutagenesis experiments to elucidate the roles of key amino acids in the multistep DH1 catalysis, identifying critical functions for leucine and tyrosine side chains. The mutagenesis results were applied to add a secondary isomerase functionality to a nonisomerizing DH in the first successful gain-of-function engineering of a PKS DH. Our studies of GphF DH1 catalysis highlight the versatility of the DH active site and adaptation for a specific catalytic outcome with a specific substrate.
中文翻译:

吉菲膦酸聚酮合酶中烯烃重排的分子基础。
聚酮合酶(PKS)是具有不同化学成分和生物学意义的天然产物的丰富来源。在这里,我们报告了吉菲膦酸(一种真核蛋白质合成抑制剂)PKS 途径中非典型脱水酶 (DH) 结构域的特征。使用合成底物模拟物库,通过 LC-MS 分析探测 GphF DH1 的反应过程、立体特异性和对非天然底物的耐受性。总而言之,这些研究确定 GphF DH1 是一种双功能脱水酶/异构酶,它安装奇偶双键并产生与吉菲膦酸异丁烯基末端一致的产物。研究还揭示了 C2 差向异构酶在与天然底物的催化周转中具有意想不到的功能。 GphF DH1 的 1.55-Å 晶体结构指导诱变实验,以阐明关键氨基酸在多步 DH1 催化中的作用,确定亮氨酸和酪氨酸侧链的关键功能。诱变结果应用于向非异构化 DH 添加二级异构酶功能,这是 PKS DH 的首次成功的功能获得工程。我们对 GphF DH1 催化的研究强调了 DH 活性位点的多功能性以及对特定底物的特定催化结果的适应性。
更新日期:2018-09-04
中文翻译:

吉菲膦酸聚酮合酶中烯烃重排的分子基础。
聚酮合酶(PKS)是具有不同化学成分和生物学意义的天然产物的丰富来源。在这里,我们报告了吉菲膦酸(一种真核蛋白质合成抑制剂)PKS 途径中非典型脱水酶 (DH) 结构域的特征。使用合成底物模拟物库,通过 LC-MS 分析探测 GphF DH1 的反应过程、立体特异性和对非天然底物的耐受性。总而言之,这些研究确定 GphF DH1 是一种双功能脱水酶/异构酶,它安装奇偶双键并产生与吉菲膦酸异丁烯基末端一致的产物。研究还揭示了 C2 差向异构酶在与天然底物的催化周转中具有意想不到的功能。 GphF DH1 的 1.55-Å 晶体结构指导诱变实验,以阐明关键氨基酸在多步 DH1 催化中的作用,确定亮氨酸和酪氨酸侧链的关键功能。诱变结果应用于向非异构化 DH 添加二级异构酶功能,这是 PKS DH 的首次成功的功能获得工程。我们对 GphF DH1 催化的研究强调了 DH 活性位点的多功能性以及对特定底物的特定催化结果的适应性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号