Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
3D microfluidic ex vivo culture of organotypic tumor spheroids to model immune checkpoint blockade
Lab on a Chip ( IF 6.1 ) Pub Date : 2018-09-05 00:00:00 , DOI: 10.1039/c8lc00322j Amir R Aref 1 , Marco Campisi , Elena Ivanova , Andrew Portell , Dalia Larios , Brandon P Piel , Natasha Mathur , Chensheng Zhou , Raven Vlahos Coakley , Alan Bartels , Michaela Bowden , Zach Herbert , Sarah Hill , Sean Gilhooley , Jacob Carter , Israel Cañadas , Tran C Thai , Shunsuke Kitajima , Valeria Chiono , Cloud P Paweletz , David A Barbie , Roger D Kamm , Russell W Jenkins
Lab on a Chip ( IF 6.1 ) Pub Date : 2018-09-05 00:00:00 , DOI: 10.1039/c8lc00322j Amir R Aref 1 , Marco Campisi , Elena Ivanova , Andrew Portell , Dalia Larios , Brandon P Piel , Natasha Mathur , Chensheng Zhou , Raven Vlahos Coakley , Alan Bartels , Michaela Bowden , Zach Herbert , Sarah Hill , Sean Gilhooley , Jacob Carter , Israel Cañadas , Tran C Thai , Shunsuke Kitajima , Valeria Chiono , Cloud P Paweletz , David A Barbie , Roger D Kamm , Russell W Jenkins
Affiliation
|
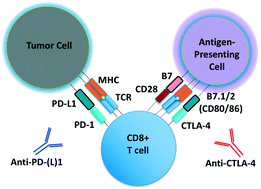
Microfluidic culture has the potential to revolutionize cancer diagnosis and therapy. Indeed, several microdevices are being developed specifically for clinical use to test novel cancer therapeutics. To be effective, these platforms need to replicate the continuous interactions that exist between tumor cells and non-tumor cell elements of the tumor microenvironment through direct cell–cell or cell–matrix contact or by the secretion of signaling factors such as cytokines, chemokines and growth factors. Given the challenges of personalized or precision cancer therapy, especially with the advent of novel immunotherapies, a critical need exists for more sophisticated ex vivo diagnostic systems that recapitulate patient-specific tumor biology with the potential to predict response to immune-based therapies in real-time. Here, we present details of a method to screen for the response of patient tumors to immune checkpoint blockade therapy, first reported in Jenkins et al. Cancer Discovery, 2018, 8, 196–215, with updated evaluation of murine- and patient-derived organotypic tumor spheroids (MDOTS/PDOTS), including evaluation of the requirement for 3D microfluidic culture in MDOTS, demonstration of immune-checkpoint sensitivity of PDOTS, and expanded evaluation of tumor–immune interactions using RNA-sequencing to infer changes in the tumor–immune microenvironment. We also examine some potential improvements to current systems and discuss the challenges in translating such diagnostic assays to the clinic.
中文翻译:

器官型肿瘤球体的 3D 微流体离体培养以模拟免疫检查点阻断
微流体培养具有彻底改变癌症诊断和治疗的潜力。实际上,正在开发几种微型设备,专门用于临床测试新的癌症治疗方法。为了有效,这些平台需要通过直接的细胞-细胞或细胞-基质接触或通过分泌信号因子(如细胞因子、趋化因子和生长因子。鉴于个性化或精准癌症治疗的挑战,特别是随着新型免疫疗法的出现,迫切需要更复杂的离体诊断系统概括了患者特异性肿瘤生物学,并有可能实时预测对基于免疫的治疗的反应。在这里,我们介绍了一种筛选患者肿瘤对免疫检查点阻断治疗反应的方法的详细信息,该方法首先在 Jenkins等人中报道。癌症发现, 2018, 8, 196–215,更新了对小鼠和患者来源的器官型肿瘤球体 (MDOTS/PDOTS) 的评估,包括评估 MDOTS 中对 3D 微流体培养的要求、证明 PDOTS 的免疫检查点敏感性以及扩大对肿瘤的评估– 使用 RNA 测序的免疫相互作用来推断肿瘤免疫微环境的变化。我们还研究了对当前系统的一些潜在改进,并讨论了将此类诊断分析应用于临床的挑战。
更新日期:2018-09-05
中文翻译:

器官型肿瘤球体的 3D 微流体离体培养以模拟免疫检查点阻断
微流体培养具有彻底改变癌症诊断和治疗的潜力。实际上,正在开发几种微型设备,专门用于临床测试新的癌症治疗方法。为了有效,这些平台需要通过直接的细胞-细胞或细胞-基质接触或通过分泌信号因子(如细胞因子、趋化因子和生长因子。鉴于个性化或精准癌症治疗的挑战,特别是随着新型免疫疗法的出现,迫切需要更复杂的离体诊断系统概括了患者特异性肿瘤生物学,并有可能实时预测对基于免疫的治疗的反应。在这里,我们介绍了一种筛选患者肿瘤对免疫检查点阻断治疗反应的方法的详细信息,该方法首先在 Jenkins等人中报道。癌症发现, 2018, 8, 196–215,更新了对小鼠和患者来源的器官型肿瘤球体 (MDOTS/PDOTS) 的评估,包括评估 MDOTS 中对 3D 微流体培养的要求、证明 PDOTS 的免疫检查点敏感性以及扩大对肿瘤的评估– 使用 RNA 测序的免疫相互作用来推断肿瘤免疫微环境的变化。我们还研究了对当前系统的一些潜在改进,并讨论了将此类诊断分析应用于临床的挑战。











































 京公网安备 11010802027423号
京公网安备 11010802027423号