当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Trifluoromethyl‐α,β‐unsaturated Lactones and Pyrazolinones and Discovery of Influenza Virus Polymerase Inhibitors
ChemMedChem ( IF 3.6 ) Pub Date : 2018-10-09 , DOI: 10.1002/cmdc.201800511 Satoshi Mizuta 1 , Juliann Nzembi Makau 2 , Ayako Kitagawa 1 , Kanami Kitamura 1 , Hiroki Otaki 1 , Kodai Nishi 3 , Ken Watanabe 2
ChemMedChem ( IF 3.6 ) Pub Date : 2018-10-09 , DOI: 10.1002/cmdc.201800511 Satoshi Mizuta 1 , Juliann Nzembi Makau 2 , Ayako Kitagawa 1 , Kanami Kitamura 1 , Hiroki Otaki 1 , Kodai Nishi 3 , Ken Watanabe 2
Affiliation
|
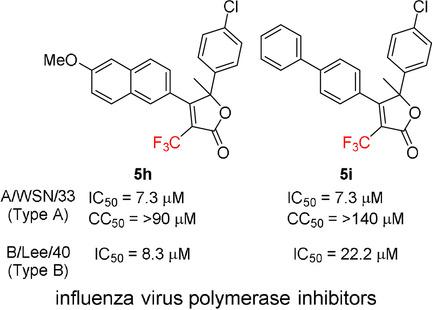
To explore the potential biological activities of trifluoromethyl heterocycles, we recently developed a synthetic approach to access a series of α‐trifluoromethyl‐α,β‐unsaturated lactones and trifluoromethyl pyrazolinones. The compounds were tested for their antimicrobial activity, and we found that some compounds had anti‐influenza viral activity. The β‐aryl‐α‐trifluoromethyl α,β‐unsaturated lactone derivatives 5 g (5‐(4‐chlorophenyl)‐5‐methyl‐4‐phenyl‐3‐(trifluoromethyl)furan‐2‐one), 7 b (4‐(4‐methoxyphenyl)‐3‐(trifluoromethyl)spiro[furan‐5,1′‐indane]‐2‐one), and the trifluoromethyl pyrazolinone 12 c (1‐(6‐methoxy‐2‐naphthyl)‐2‐(trifluoromethyl)‐5,6,7,8‐tetrahydropyrazolo[1,2‐a]pyridazin‐3‐one) were found to possess promising inhibitory activity against influenza virus type A, strain A/WSN/33 (H1N1). These three hit compounds were successfully optimized, and we identified that the most potent compound 5 h (5‐(4‐chlorophenyl)‐4‐(6‐methoxy‐2‐naphthyl)‐5‐methyl‐3‐(trifluoromethyl)furan‐2‐one) showed inhibitory activity against various types of influenza A and B viruses in the low‐micromolar range without showing cytotoxicity. Moreover, 5 h was more effective against the clinical isolate A/California/7/2009 (H1N1pdm) strain than the influenza viral polymerase inhibitor, favipiravir (T‐705). We also delineated the structure–activity relationship and obtained mechanistic insight into inhibition of the viral polymerase.
中文翻译:

三氟甲基-α,β-不饱和内酯和吡唑啉酮的合成及流感病毒聚合酶抑制剂的发现
为了探索三氟甲基杂环的潜在生物活性,我们最近开发了一种合成方法来获得一系列α-三氟甲基-α,β-不饱和内酯和三氟甲基吡唑啉酮。测试了这些化合物的抗微生物活性,我们发现某些化合物具有抗流感病毒活性。β-芳基-α-三氟甲基α,β-不饱和内酯衍生物5 g(5-(4-氯苯基)-5-甲基-4-苯基-3-(三氟甲基)呋喃-2-一),7 b(4 -(4-甲氧基苯基)-3-(三氟甲基)螺[呋喃-5,1'-茚满] -2-酮)和三氟甲基吡唑啉酮12 c(1-(6-甲氧基-2-萘基)-2-(三氟甲基)-5,6,7,8-四氢吡唑并[1,2-a]哒嗪-3-一)被发现对流感具有抑制作用A型病毒,菌株A / WSN / 33(H1N1)。成功地优化了这三种命中化合物,我们确定了最有效的化合物5小时(5-(4-氯苯基)-4-(6-甲氧基-2-萘基)-5-甲基-3-(三氟甲基)呋喃2‐1)在低微摩尔范围内显示出对各种类型的甲型和乙型流感病毒的抑制活性,而无细胞毒性。而且5小时与流感病毒聚合酶抑制剂favipiravir(T-705)相比,对临床分离株A / California / 7/2009(H1N1pdm)菌株更有效。我们还描述了结构与活性之间的关系,并获得了对病毒聚合酶抑制作用的机械学见解。
更新日期:2018-10-09
中文翻译:

三氟甲基-α,β-不饱和内酯和吡唑啉酮的合成及流感病毒聚合酶抑制剂的发现
为了探索三氟甲基杂环的潜在生物活性,我们最近开发了一种合成方法来获得一系列α-三氟甲基-α,β-不饱和内酯和三氟甲基吡唑啉酮。测试了这些化合物的抗微生物活性,我们发现某些化合物具有抗流感病毒活性。β-芳基-α-三氟甲基α,β-不饱和内酯衍生物5 g(5-(4-氯苯基)-5-甲基-4-苯基-3-(三氟甲基)呋喃-2-一),7 b(4 -(4-甲氧基苯基)-3-(三氟甲基)螺[呋喃-5,1'-茚满] -2-酮)和三氟甲基吡唑啉酮12 c(1-(6-甲氧基-2-萘基)-2-(三氟甲基)-5,6,7,8-四氢吡唑并[1,2-a]哒嗪-3-一)被发现对流感具有抑制作用A型病毒,菌株A / WSN / 33(H1N1)。成功地优化了这三种命中化合物,我们确定了最有效的化合物5小时(5-(4-氯苯基)-4-(6-甲氧基-2-萘基)-5-甲基-3-(三氟甲基)呋喃2‐1)在低微摩尔范围内显示出对各种类型的甲型和乙型流感病毒的抑制活性,而无细胞毒性。而且5小时与流感病毒聚合酶抑制剂favipiravir(T-705)相比,对临床分离株A / California / 7/2009(H1N1pdm)菌株更有效。我们还描述了结构与活性之间的关系,并获得了对病毒聚合酶抑制作用的机械学见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号