当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Gold-catalyzed annulations of N-aryl ynamides with benzisoxazoles to construct 6H-indolo[2,3-b]quinoline cores†
Chemical Communications ( IF 4.9 ) Pub Date : 2018-09-04 00:00:00 , DOI: 10.1039/c8cc04264k Meng-Han Tsai 1, 2, 3, 4 , Cheng-Yu Wang 1, 2, 3, 4, 5 , Antony Sekar Kulandai Raj 1, 2, 3, 4 , Rai-Shung Liu 1, 2, 3, 4
Chemical Communications ( IF 4.9 ) Pub Date : 2018-09-04 00:00:00 , DOI: 10.1039/c8cc04264k Meng-Han Tsai 1, 2, 3, 4 , Cheng-Yu Wang 1, 2, 3, 4, 5 , Antony Sekar Kulandai Raj 1, 2, 3, 4 , Rai-Shung Liu 1, 2, 3, 4
Affiliation
|
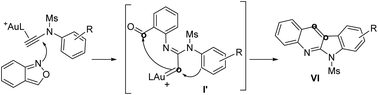
This work reports new annulations of N-aryl ynamides with benzisoxazoles to form 6H-indolo[2,3-b]quinoline derivatives. The synthetic utility of this new method is manifested by its applicability to access naturally occurring alkaloids including norcryptotackeine, neocryptolepine and 11-methylneocryptol-epine. Our experimental data indicate that high-temperature conditions allow N-aryl nucleophiles to become conformationally flexible, rendering the attack at gold carbenes effective to generate reactive indoles that attack again the benzaldehyde to furnish the observed products.
中文翻译:

金催化的N-芳基酰胺与苯并异恶唑的环化反应,构建6个H-吲哚[2,3- b ]喹啉核†
这项工作报告N-芳基乙酰胺与苯并异恶唑的新环化反应形成6 H-吲哚[2,3- b ]喹啉衍生物。这种新方法的合成效用体现在其可利用天然存在的生物碱,包括去甲肾上腺素,新甲氧肾上腺素和11-甲基新甲氧肾上腺素中。我们的实验数据表明,高温条件可使N-芳基亲核试剂具有构象柔性,从而使对金卡宾的攻击有效地产生了反应性吲哚,这些吲哚又再次攻击了苯甲醛以提供所观察到的产物。
更新日期:2018-09-04
中文翻译:

金催化的N-芳基酰胺与苯并异恶唑的环化反应,构建6个H-吲哚[2,3- b ]喹啉核†
这项工作报告N-芳基乙酰胺与苯并异恶唑的新环化反应形成6 H-吲哚[2,3- b ]喹啉衍生物。这种新方法的合成效用体现在其可利用天然存在的生物碱,包括去甲肾上腺素,新甲氧肾上腺素和11-甲基新甲氧肾上腺素中。我们的实验数据表明,高温条件可使N-芳基亲核试剂具有构象柔性,从而使对金卡宾的攻击有效地产生了反应性吲哚,这些吲哚又再次攻击了苯甲醛以提供所观察到的产物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号