Microporous and Mesoporous Materials ( IF 4.8 ) Pub Date : 2018-09-04 , DOI: 10.1016/j.micromeso.2018.08.035 D.A. Kennedy , M. Mujčin , C. Abou-Zeid , F.H. Tezel
|
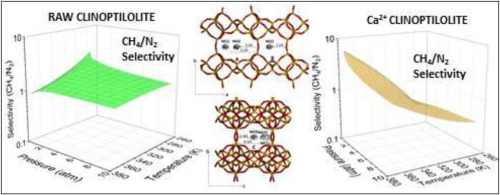
This study details an experimental analysis of the thermodynamics of adsorption equilibrium behaviour of pure CO2, CH4, and N2 gases on raw, Fe3+, Ca2+, and Cs+ cation exchanged clinoptilolite. Equilibrium adsorption isotherms were experimentally determined for CO2, CH4, and N2 at three different temperatures ranging from 303 K up to 363 K, at pressures up to 8.0atm using the microgravimetric technique. Kinetic adsorption uptake data was measured at different temperatures for CH4 and N2 gases, and the results were compared for raw and Ca2+ clinoptilolite. Heat of adsorption values were calculated for all three gases on the studied clinoptilolite samples using the chromatographic method. These results were compared to the isosteric adsorption enthalpies determined from the adsorption isotherms at different temperatures. The isosteric heat of adsorption was the highest for CO2 followed by CH4, and N2. Temperature dependent Sips, Toth, and Dual-Site Langmuir isotherm models were fitted with experimental data and selectivity factor values were calculated for CO2/CH4 and CH4/N2 gas separations using the regressed isotherm models. Ca2+ clinoptilolite shows the best potential for CO2/CH4 separations due to its high selectivity. However, higher temperatures are needed in order to compensate for the slow kinetic behaviour. For CH4/N2 separations, Fe3+ clinoptilolite selectivity displays both high values and less variability compared to Cs+ clinoptilolite over a broader range of temperatures and pressures.
中文翻译:

斜发沸石的阳离子交换改性-热力学对二氧化碳,甲烷和氮的吸附分离的影响
这项研究详细分析了纯CO 2,CH 4和N 2气体在原料,Fe 3+,Ca 2+和Cs +阳离子交换斜发沸石上的吸附平衡行为的热力学实验分析。使用微重力技术,在303 K至363 K的三个不同温度,压力高达8.0atm的条件下,通过实验确定了CO 2,CH 4和N 2的平衡吸附等温线。测量了在不同温度下CH 4和N 2气体的动力学吸附吸收数据,并对原始和Ca 2+的结果进行了比较。斜发沸石。使用色谱方法,计算了研究的斜发沸石样品上所有三种气体的吸附热值。将这些结果与由在不同温度下的吸附等温线确定的等排吸附焓进行了比较。对于CO 2,其等排吸附热最高,其次是CH 4和N 2。温度相关的Sips,Toth和Dual-Site Langmuir等温线模型拟合了实验数据,并使用回归等温线模型计算了CO 2 / CH 4和CH 4 / N 2气体分离的选择性因子值。钙2+斜发沸石由于其高选择性而显示出最佳的CO 2 / CH 4分离潜力。但是,需要较高的温度以补偿缓慢的动力学行为。对于CH 4 / N 2分离,在更宽的温度和压力范围内,与Cs +斜发沸石相比,Fe 3+斜发沸石的选择性显示出较高的值和较小的变异性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号