Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Microfluidic affinity separation chip for selective capture and release of label-free ovarian cancer exosomes†
Lab on a Chip ( IF 6.1 ) Pub Date : 2018-09-04 00:00:00 , DOI: 10.1039/c8lc00834e Colin L. Hisey 1, 2, 3, 4 , Kalpana Deepa Priya Dorayappan 3, 4, 5, 6, 7 , David E. Cohn 3, 4, 5, 6, 7 , Karuppaiyah Selvendiran 3, 4, 5, 6, 7 , Derek J. Hansford 1, 2, 3, 4
Lab on a Chip ( IF 6.1 ) Pub Date : 2018-09-04 00:00:00 , DOI: 10.1039/c8lc00834e Colin L. Hisey 1, 2, 3, 4 , Kalpana Deepa Priya Dorayappan 3, 4, 5, 6, 7 , David E. Cohn 3, 4, 5, 6, 7 , Karuppaiyah Selvendiran 3, 4, 5, 6, 7 , Derek J. Hansford 1, 2, 3, 4
Affiliation
|
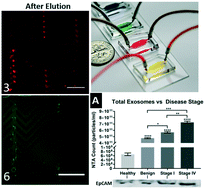
Exosomes are nanoscale vesicles found in many bodily fluids which play a significant role in cell-to-cell signaling and contain biomolecules indicative of their cells of origin. Recently, microfluidic devices have provided the ability to efficiently capture exosomes based on specific membrane biomarkers, but releasing the captured exosomes intact and label-free for downstream characterization and experimentation remains a challenge. We present a herringbone-grooved microfluidic device which is covalently functionalized with antibodies against general and cancer exosome membrane biomarkers (CD9 and EpCAM) to isolate exosomes from small volumes of high-grade serous ovarian cancer (HGSOC) serum. Following capture, intact exosomes are released label-free using a low pH buffer and immediately neutralized downstream to ensure their stability. Characterization of captured and released exosomes was performed using fluorescence microscopy, nanoparticle tracking analysis, flow-cytometry, and SEM. Our results demonstrate the successful isolation of intact and label-free exosomes, indicate that the amount of both total and EpCAM+ exosomes increases with HGSOC disease progression, and demonstrate the downstream internalization of isolated exosomes by OVCAR8 cells. This device and approach can be utilized for a nearly limitless range of downstream exosome analytical and experimental techniques, both on and off-chip.
中文翻译:

微流亲和分离芯片可选择性捕获和释放无标签的卵巢癌外泌体†
外泌体是在许多体液中发现的纳米级囊泡,它们在细胞间信号传导中起重要作用,并含有指示其起源细胞的生物分子。近来,微流体装置已经提供了基于特定膜生物标记物有效捕获外泌体的能力,但是完整地释放捕获的外泌体且无标签以用于下游表征和实验仍然是一个挑战。我们提出了一种人字形的微流控设备,该设备与针对一般和癌症外泌体膜生物标志物(CD9和EpCAM)的抗体共价功能化,以从少量高级别浆液性卵巢癌(HGSOC)血清中分离外泌体。捕获后,使用低pH缓冲液将完整的外泌体释放无标签,并立即在下游中和以确保其稳定性。使用荧光显微镜,纳米粒子跟踪分析,流式细胞仪和SEM对捕获和释放的外泌体进行表征。我们的结果证明了完整和无标记外泌体的成功分离,表明总外泌体和EpCAM +外泌体的数量均随着HGSOC疾病进展而增加,并证明了OVCAR8细胞对分离的外泌体的下游内在化。这种装置和方法可用于芯片上和芯片外几乎无限范围的下游外泌体分析和实验技术。提示总的和EpCAM +外泌体的数量都随着HGSOC疾病的进展而增加,并证明了OVCAR8细胞对分离的外泌体的下游内在化。这种装置和方法可用于芯片上和芯片外几乎无限范围的下游外泌体分析和实验技术。表明总的和EpCAM +外泌体的数量都随着HGSOC疾病进展而增加,并证明了OVCAR8细胞对分离的外泌体的下游内在化。这种装置和方法可用于芯片上和芯片外几乎无限范围的下游外泌体分析和实验技术。
更新日期:2018-09-04
中文翻译:

微流亲和分离芯片可选择性捕获和释放无标签的卵巢癌外泌体†
外泌体是在许多体液中发现的纳米级囊泡,它们在细胞间信号传导中起重要作用,并含有指示其起源细胞的生物分子。近来,微流体装置已经提供了基于特定膜生物标记物有效捕获外泌体的能力,但是完整地释放捕获的外泌体且无标签以用于下游表征和实验仍然是一个挑战。我们提出了一种人字形的微流控设备,该设备与针对一般和癌症外泌体膜生物标志物(CD9和EpCAM)的抗体共价功能化,以从少量高级别浆液性卵巢癌(HGSOC)血清中分离外泌体。捕获后,使用低pH缓冲液将完整的外泌体释放无标签,并立即在下游中和以确保其稳定性。使用荧光显微镜,纳米粒子跟踪分析,流式细胞仪和SEM对捕获和释放的外泌体进行表征。我们的结果证明了完整和无标记外泌体的成功分离,表明总外泌体和EpCAM +外泌体的数量均随着HGSOC疾病进展而增加,并证明了OVCAR8细胞对分离的外泌体的下游内在化。这种装置和方法可用于芯片上和芯片外几乎无限范围的下游外泌体分析和实验技术。提示总的和EpCAM +外泌体的数量都随着HGSOC疾病的进展而增加,并证明了OVCAR8细胞对分离的外泌体的下游内在化。这种装置和方法可用于芯片上和芯片外几乎无限范围的下游外泌体分析和实验技术。表明总的和EpCAM +外泌体的数量都随着HGSOC疾病进展而增加,并证明了OVCAR8细胞对分离的外泌体的下游内在化。这种装置和方法可用于芯片上和芯片外几乎无限范围的下游外泌体分析和实验技术。











































 京公网安备 11010802027423号
京公网安备 11010802027423号