当前位置:
X-MOL 学术
›
Arab. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A new copper(II) chelate complex with polyamines as fire retardant and epoxy hardener: Synthesis, crystal and electronic structure, and thermal behavior of (ethylenediamine-N, N')-(diethylenetriamine-N, N', N'')-copper(II) hexafluoridosilicate
Arabian Journal of Chemistry ( IF 5.3 ) Pub Date : 2020-01-01 , DOI: 10.1016/j.arabjc.2018.08.014 Helen Lavrenyuk , Borys Mykhalichko , Błażej Dziuk , Volodymyr Olijnyk , Oleg Mykhalichko
Arabian Journal of Chemistry ( IF 5.3 ) Pub Date : 2020-01-01 , DOI: 10.1016/j.arabjc.2018.08.014 Helen Lavrenyuk , Borys Mykhalichko , Błażej Dziuk , Volodymyr Olijnyk , Oleg Mykhalichko
|
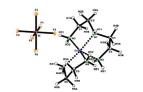
Abstract A new (ethylenediamine-N,N′)-(diethylenetriamine-N,N′,N″)-copper(II) hexafluoridosilicate complex, [Cu(eda)(deta)]SiF6 (1) (eda – ethylenediamine; deta – diethylenetriamine), was synthesized by direct interaction of anhydrous CuSiF6 with polyethylenepolyamine (pepa – H2N[ C2H4NH ]nH, where n = 1 (eda) and 2 (deta)). The crystals of 1 were characterized by IR spectroscopy and X-ray diffraction. Compound 1 consists of SiF62− discrete anions and [Cu(eda)(deta)]2+ complex cations whose Cu2+ ions are chelated by eda and deta. The coordination polyhedron of Cu(II) atom is an elongated square pyramid which consists of four nitrogen atoms belonging to NH2 groups of eda and NH2 and NH groups of deta at the base of the pyramid and of one more nitrogen atom from NH2 group of deta at its apical position. Ab initio quantum-chemical calculations of chelation process were carried out for 1 by the restricted Hartree-Fock method using a 6-31G∗ basis set. The influence of the chelate complex 1 upon its fire retardant properties was analyzed. The calculated electron-stereo-chemical parameters for 1 are in a good agreement with its thermal parameters investigated by differential thermal analysis. The thermal decomposition of 1 is finished off at 368 °C and the maximal combustion temperature of gaseous decomposition products is 544 °C.
中文翻译:

一种新的铜(II)螯合物与多胺作为阻燃剂和环氧固化剂:(乙二胺-N,N')-(二亚乙基三胺-N,N',N'')-的合成、晶体和电子结构以及热行为六氟硅酸铜(II)
摘要 一种新的 (乙二胺-N,N')-(二亚乙基三胺-N,N',N″)-铜(II) 六氟硅酸盐络合物,[Cu(eda)(deta)]SiF6 (1) (eda – 乙二胺; deta – 二亚乙基三胺),通过无水 CuSiF6 与聚乙烯多胺(pepa – H2N[ C2H4NH ]nH,其中 n = 1 (eda) 和 2 (deta))的直接相互作用合成。1的晶体通过红外光谱和X射线衍射表征。化合物 1 由 SiF62- 离散阴离子和 [Cu(eda)(deta)]2+ 复合阳离子组成,其 Cu2+ 离子被 eda 和 deta 螯合。Cu(II)原子的配位多面体是一个细长的方形金字塔,它由四个属于 eda 和 NH2 的氮原子和位于金字塔底部的 deta 的 NH 基团和另外一个来自 NH2 的 deta 基团的氮原子组成。在它的顶端位置。螯合过程的从头算量子化学计算是通过限制性 Hartree-Fock 方法使用 6-31G* 基组对 1 进行的。分析了螯合物1对其阻燃性能的影响。1 的计算电子立体化学参数与其通过差热分析研究的热参数非常一致。1 的热分解在 368°C 完成,气态分解产物的最高燃烧温度为 544°C。1 的计算电子立体化学参数与其通过差热分析研究的热参数非常一致。1 的热分解在 368°C 完成,气态分解产物的最高燃烧温度为 544°C。1 的计算电子立体化学参数与其通过差热分析研究的热参数非常一致。1 的热分解在 368°C 完成,气态分解产物的最高燃烧温度为 544°C。
更新日期:2020-01-01
中文翻译:

一种新的铜(II)螯合物与多胺作为阻燃剂和环氧固化剂:(乙二胺-N,N')-(二亚乙基三胺-N,N',N'')-的合成、晶体和电子结构以及热行为六氟硅酸铜(II)
摘要 一种新的 (乙二胺-N,N')-(二亚乙基三胺-N,N',N″)-铜(II) 六氟硅酸盐络合物,[Cu(eda)(deta)]SiF6 (1) (eda – 乙二胺; deta – 二亚乙基三胺),通过无水 CuSiF6 与聚乙烯多胺(pepa – H2N[ C2H4NH ]nH,其中 n = 1 (eda) 和 2 (deta))的直接相互作用合成。1的晶体通过红外光谱和X射线衍射表征。化合物 1 由 SiF62- 离散阴离子和 [Cu(eda)(deta)]2+ 复合阳离子组成,其 Cu2+ 离子被 eda 和 deta 螯合。Cu(II)原子的配位多面体是一个细长的方形金字塔,它由四个属于 eda 和 NH2 的氮原子和位于金字塔底部的 deta 的 NH 基团和另外一个来自 NH2 的 deta 基团的氮原子组成。在它的顶端位置。螯合过程的从头算量子化学计算是通过限制性 Hartree-Fock 方法使用 6-31G* 基组对 1 进行的。分析了螯合物1对其阻燃性能的影响。1 的计算电子立体化学参数与其通过差热分析研究的热参数非常一致。1 的热分解在 368°C 完成,气态分解产物的最高燃烧温度为 544°C。1 的计算电子立体化学参数与其通过差热分析研究的热参数非常一致。1 的热分解在 368°C 完成,气态分解产物的最高燃烧温度为 544°C。1 的计算电子立体化学参数与其通过差热分析研究的热参数非常一致。1 的热分解在 368°C 完成,气态分解产物的最高燃烧温度为 544°C。











































 京公网安备 11010802027423号
京公网安备 11010802027423号