当前位置:
X-MOL 学术
›
Cell Chem. Bio.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selenocysteine-Specific Mass Spectrometry Reveals Tissue-Distinct Selenoproteomes and Candidate Selenoproteins
Cell Chemical Biology ( IF 6.6 ) Pub Date : 2018-08-30 , DOI: 10.1016/j.chembiol.2018.08.006 Lin Guo , Wu Yang , Qiang Huang , Jiali Qiang , Jonathan Ross Hart , Wenyuan Wang , Junhao Hu , Jidong Zhu , Nan Liu , Yaoyang Zhang
Cell Chemical Biology ( IF 6.6 ) Pub Date : 2018-08-30 , DOI: 10.1016/j.chembiol.2018.08.006 Lin Guo , Wu Yang , Qiang Huang , Jiali Qiang , Jonathan Ross Hart , Wenyuan Wang , Junhao Hu , Jidong Zhu , Nan Liu , Yaoyang Zhang
|
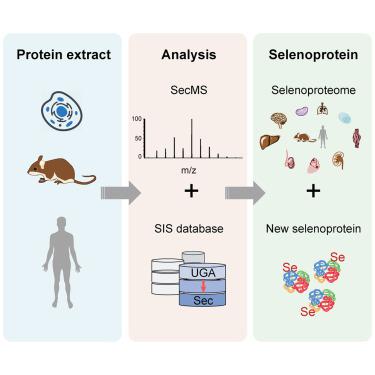
Selenoproteins, defined by the presence of selenocysteines (Sec), play important roles in a wide range of biological processes. All known selenoproteins are marked by the presence of Sec insertion sequence (SECIS) at their mRNA. The lack of an effective analytical method has hindered our ability to explore the selenoproteome and new selenoproteins beyond SECIS. Here, we develop a Sec-specific mass spectrometry-based technique, termed “SecMS,” which allows the systematic profiling of selenoproteomes by selective alkylation of Sec. Using SecMS, we quantitatively characterized the age- and stress-regulated selenoproteomes for nine tissues from mice of different ages and mammalian cells, demonstrating tissue-specific selenoproteomes and an age-dependent decline in specific selenoproteins in brains and hearts. We established an integrated platform using SecMS and SECIS-independent selenoprotein (SIS) database and further identified five candidate selenoproteins. The application of this integrated platform provides an effective strategy to explore the selenoproteome independent of SECIS.
中文翻译:

硒代半胱氨酸特异性质谱法揭示了组织不同的硒代蛋白质组和候选硒代蛋白
硒代半胱氨酸(Sec)的存在定义了硒蛋白,在广泛的生物学过程中起着重要的作用。所有已知的硒蛋白都通过其mRNA上存在Sec插入序列(SECIS)进行标记。缺乏有效的分析方法阻碍了我们探索SECIS以外的硒蛋白组和新硒蛋白的能力。在这里,我们开发了一种基于Sec质谱的特定技术,称为“ SecMS”,该技术可通过对Sec进行选择性烷基化来对硒蛋白组进行系统分析。使用SecMS,我们定量分析了来自不同年龄小鼠和哺乳动物细胞的9种组织的年龄和压力调节的硒蛋白组,证明了组织特异性硒蛋白组和脑和心脏中特定硒蛋白的年龄依赖性下降。我们使用SecMS和不依赖SECIS的硒蛋白(SIS)数据库建立了一个集成平台,并进一步确定了五种候选硒蛋白。该集成平台的应用为探索独立于SECIS的硒蛋白组提供了有效的策略。
更新日期:2018-11-15
中文翻译:

硒代半胱氨酸特异性质谱法揭示了组织不同的硒代蛋白质组和候选硒代蛋白
硒代半胱氨酸(Sec)的存在定义了硒蛋白,在广泛的生物学过程中起着重要的作用。所有已知的硒蛋白都通过其mRNA上存在Sec插入序列(SECIS)进行标记。缺乏有效的分析方法阻碍了我们探索SECIS以外的硒蛋白组和新硒蛋白的能力。在这里,我们开发了一种基于Sec质谱的特定技术,称为“ SecMS”,该技术可通过对Sec进行选择性烷基化来对硒蛋白组进行系统分析。使用SecMS,我们定量分析了来自不同年龄小鼠和哺乳动物细胞的9种组织的年龄和压力调节的硒蛋白组,证明了组织特异性硒蛋白组和脑和心脏中特定硒蛋白的年龄依赖性下降。我们使用SecMS和不依赖SECIS的硒蛋白(SIS)数据库建立了一个集成平台,并进一步确定了五种候选硒蛋白。该集成平台的应用为探索独立于SECIS的硒蛋白组提供了有效的策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号