当前位置:
X-MOL 学术
›
ChemSusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reactivity Determinants in Electrodeposited Cu Foams for Electrochemical CO2 Reduction
ChemSusChem ( IF 7.5 ) Pub Date : 2018-08-30 , DOI: 10.1002/cssc.201801582 Katharina Klingan 1 , Tintula Kottakkat 2 , Zarko P. Jovanov 3 , Shan Jiang 1 , Chiara Pasquini 1 , Fabian Scholten 4 , Paul Kubella 1 , Arno Bergmann 3, 5 , Beatriz Roldan Cuenya 4, 5 , Christina Roth 2 , Holger Dau 1
ChemSusChem ( IF 7.5 ) Pub Date : 2018-08-30 , DOI: 10.1002/cssc.201801582 Katharina Klingan 1 , Tintula Kottakkat 2 , Zarko P. Jovanov 3 , Shan Jiang 1 , Chiara Pasquini 1 , Fabian Scholten 4 , Paul Kubella 1 , Arno Bergmann 3, 5 , Beatriz Roldan Cuenya 4, 5 , Christina Roth 2 , Holger Dau 1
Affiliation
|
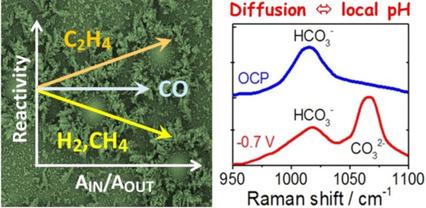
CO2 reduction is of significant interest for the production of nonfossil fuels. The reactivity of eight Cu foams with substantially different morphologies was comprehensively investigated by analysis of the product spectrum and in situ electrochemical spectroscopies (X‐ray absorption near edge structure, extended X‐ray absorption fine structure, X‐ray photoelectron spectroscopy, and Raman spectroscopy). The approach provided new insight into the reactivity determinants: The morphology, stable Cu oxide phases, and *CO poisoning of the H2 formation reaction are not decisive; the electrochemically active surface area influences the reactivity trends; macroscopic diffusion limits the proton supply, resulting in pronounced alkalization at the CuCat surfaces (operando Raman spectroscopy). H2 and CH4 formation was suppressed by macroscopic buffer alkalization, whereas CO and C2H4 formation still proceeded through a largely pH‐independent mechanism. C2H4 was formed from two CO precursor species, namely adsorbed *CO and dissolved CO present in the foam cavities.
中文翻译:

电沉积铜泡沫中电化学还原CO2的反应性决定因素
减少CO 2对于生产非化石燃料具有重大意义。通过分析产品光谱和原位电化学光谱(边缘结构附近的X射线吸收,扩展X射线吸收精细结构,X射线光电子能谱和拉曼光谱)分析了八种形态迥异的Cu泡沫的反应性。 )。该方法为反应性决定因素提供了新的见解:H 2形成反应的形态,稳定的Cu氧化物相和* CO中毒不是决定性的;电化学活性表面积影响反应性趋势;宏观扩散限制了质子的供应,从而导致CuCat表面的明显碱化(操作拉曼光谱)。H2和CH 4的形成被宏观缓冲碱化所抑制,而CO和C 2 H 4的形成仍通过很大程度上与pH无关的机制进行。C 2 H 4由两种CO前体物质形成,即泡沫腔中存在的吸附的* CO和溶解的CO。
更新日期:2018-08-30
中文翻译:

电沉积铜泡沫中电化学还原CO2的反应性决定因素
减少CO 2对于生产非化石燃料具有重大意义。通过分析产品光谱和原位电化学光谱(边缘结构附近的X射线吸收,扩展X射线吸收精细结构,X射线光电子能谱和拉曼光谱)分析了八种形态迥异的Cu泡沫的反应性。 )。该方法为反应性决定因素提供了新的见解:H 2形成反应的形态,稳定的Cu氧化物相和* CO中毒不是决定性的;电化学活性表面积影响反应性趋势;宏观扩散限制了质子的供应,从而导致CuCat表面的明显碱化(操作拉曼光谱)。H2和CH 4的形成被宏观缓冲碱化所抑制,而CO和C 2 H 4的形成仍通过很大程度上与pH无关的机制进行。C 2 H 4由两种CO前体物质形成,即泡沫腔中存在的吸附的* CO和溶解的CO。











































 京公网安备 11010802027423号
京公网安备 11010802027423号