当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Contribution of the Covalent Component of the Hydrogen-Bond Network to the Properties of Liquid Water
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-08-29 00:00:00 , DOI: 10.1021/acs.jpca.8b06857 Yifei Shi 1 , Hayden Scheiber 1 , Rustam Z. Khaliullin 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-08-29 00:00:00 , DOI: 10.1021/acs.jpca.8b06857 Yifei Shi 1 , Hayden Scheiber 1 , Rustam Z. Khaliullin 1
Affiliation
|
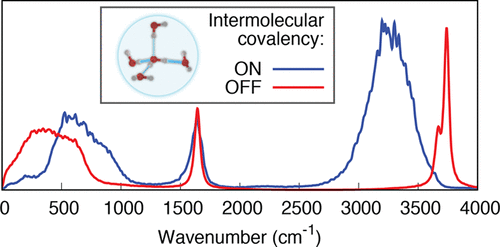
Many remarkable properties of liquid water originate from the ability of its molecules to form hydrogen bonds, each of which emerges as a combination of electrostatic, polarization, dispersion, and donor–acceptor or covalent interactions. In this work, ab initio molecular dynamics was tailored to isolate and switch off the covalent component of interactions between water molecules in simulations. Comparison of simulations with and without covalency shows that a small amount of intermolecular electron density transfer has a profound effect on the structure and dynamics of the hydrogen-bond network and thus on observable properties of room-temperature liquid water.
中文翻译:

氢键网络的共价成分对液态水性能的贡献
液态水的许多显着特性源自其分子形成氢键的能力,每个氢键都以静电,极化,分散和施主-受主或共价相互作用的组合形式出现。在这项工作中,量身定制了从头算分子动力学,以隔离和关闭模拟中水分子之间相互作用的共价成分。具有或没有共价关系的模拟的比较表明,少量的分子间电子密度转移对氢键网络的结构和动力学具有深远的影响,从而对室温液态水的可观察性产生深远的影响。
更新日期:2018-08-29
中文翻译:

氢键网络的共价成分对液态水性能的贡献
液态水的许多显着特性源自其分子形成氢键的能力,每个氢键都以静电,极化,分散和施主-受主或共价相互作用的组合形式出现。在这项工作中,量身定制了从头算分子动力学,以隔离和关闭模拟中水分子之间相互作用的共价成分。具有或没有共价关系的模拟的比较表明,少量的分子间电子密度转移对氢键网络的结构和动力学具有深远的影响,从而对室温液态水的可观察性产生深远的影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号