当前位置:
X-MOL 学术
›
Chem. Asian J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cationic Chiral Pd‐Catalyzed “Acetylenic” Diels–Alder Reaction: Computational Analysis of Reversal in Enantioselectivity
Chemistry - An Asian Journal ( IF 3.5 ) Pub Date : 2018-09-19 , DOI: 10.1002/asia.201801035 Kazuya Honda 1 , Shun Ohkura 1 , Yoshihiro Hayashi 1 , Susumu Kawauchi 1 , Koichi Mikami 1
Chemistry - An Asian Journal ( IF 3.5 ) Pub Date : 2018-09-19 , DOI: 10.1002/asia.201801035 Kazuya Honda 1 , Shun Ohkura 1 , Yoshihiro Hayashi 1 , Susumu Kawauchi 1 , Koichi Mikami 1
Affiliation
|
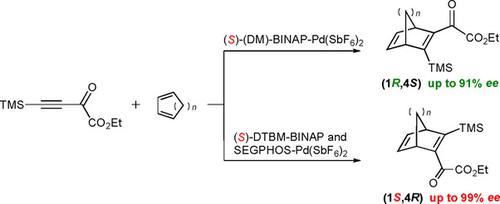
The highly enantioselective Diels–Alder reaction of acetylenic dienophiles is shown to be effectively catalyzed by cationic chiral palladium complexes. Not only the degree but also the sense of enantioselectivity critically depends on the steric demand of ligands. Computational analyses indicate that the steric demand does not affect the endo/exo‐selectivity but the enantioface selectivity of dienes.
中文翻译:

阳离子手性钯催化的“乙炔” Diels-Alder反应:对映选择性逆转的计算分析
乙二烯亲二烯体的高对映选择性Diels-Alder反应被阳离子手性钯配合物有效催化。不仅程度,而且对映选择性的感觉也严格取决于配体的空间需求。计算分析表明,空间需求量不会影响二烯的内/外选择性,但会影响二烯的对映体选择性。
更新日期:2018-09-19
中文翻译:

阳离子手性钯催化的“乙炔” Diels-Alder反应:对映选择性逆转的计算分析
乙二烯亲二烯体的高对映选择性Diels-Alder反应被阳离子手性钯配合物有效催化。不仅程度,而且对映选择性的感觉也严格取决于配体的空间需求。计算分析表明,空间需求量不会影响二烯的内/外选择性,但会影响二烯的对映体选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号