当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interactions of Selective Serotonin Reuptake Inhibitors with β-Amyloid.
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2018-09-11 , DOI: 10.1021/acschemneuro.8b00160 Gary Tin 1 , Tarek Mohamed 1 , Arash Shakeri 1 , Amy Trinh Pham 1 , Praveen P N Rao 1
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2018-09-11 , DOI: 10.1021/acschemneuro.8b00160 Gary Tin 1 , Tarek Mohamed 1 , Arash Shakeri 1 , Amy Trinh Pham 1 , Praveen P N Rao 1
Affiliation
|
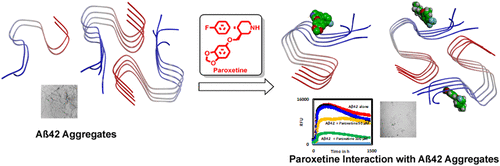
Treating Alzheimer's disease (AD) is a major challenge at the moment with no new drugs available to cure this devastating neurodegenerative disorder. In this regard, drug repurposing, which aims to determine novel therapeutic usage for drugs already approved by the regulatory agencies, is a pragmatic approach to discover novel treatment strategies. Selective serotonin reuptake inhibitors (SSRIs) are a known class of United States Food and Drug Administration approved drugs used in the treatment of depression. We investigated the ability of SSRIs fluvoxamine, fluoxetine, paroxetine, sertraline, and escitalopram on Aβ42 aggregation and fibrillogenesis. Remarkably, the aggregation kinetic experiments carried out demonstrate the anti-Aβ42 aggregation activity of SSRIs fluoxetine, paroxetine, and sertraline at all the tested concentrations (1, 10, 50, and 100 μM). Both fluoxetine and paroxetine were identified as the most promising SSRIs, showing 74.8 and 76% inhibition of Aβ42 aggregation at 100 μM. The transmission electron microscopy experiments and dot-blot study also demonstrate the ability of fluoxetine and paroxetine to prevent Aβ42 aggregation and fibrillogenesis, providing further evidence. Investigating the binding interactions of fluoxetine and paroxetine in the Aβ42 oligomer and fibril models derived from the solid-state NMR structure suggests that these SSRIs interact at a region close to the N-terminal (Lys16-Glu22) in the S-shaped cross-β-strand assembly and reduce Aβ42 fibrillogenesis. On the basis of this study, a pharmacophore model is proposed which shows that the minimum structural requirements to design novel Aβ42 aggregation inhibitors include the presence of one ionizable group, one hydrophobic group, two aromatic rings, and two hydrogen bond donor groups. These studies demonstrate that SSRIs have the potential to prevent Aβ42 aggregation by direct binding and could be beneficial to AD patients on SSRIs.
中文翻译:

选择性5-羟色胺再摄取抑制剂与β-淀粉样蛋白的相互作用。
目前尚无用于治疗这种破坏性神经退行性疾病的新药,这是治疗阿尔茨海默氏病(AD)的主要挑战。在这方面,旨在确定监管机构已经批准的药物的新颖治疗用途的药物再利用是发现新颖治疗策略的实用方法。选择性5-羟色胺再摄取抑制剂(SSRIs)是美国食品药品监督管理局批准的用于治疗抑郁症的已知药物。我们研究了SSRIs氟伏沙明,氟西汀,帕罗西汀,舍曲林和依他普仑对Aβ42聚集和原纤维形成的能力。值得注意的是,进行的聚集动力学实验表明,在所有测试浓度下,SSRIs氟西汀,帕罗西汀和舍曲林的抗Aβ42聚集活性(1、10,50和100μM)。氟西汀和帕罗西汀均被认为是最有前途的SSRI,在100μM浓度下对Aβ42聚集的抑制率为74.8和76%。透射电子显微镜实验和斑点印迹研究还证明了氟西汀和帕罗西汀具有预防Aβ42聚集和原纤维形成的能力,提供了进一步的证据。在源自固态NMR结构的Aβ42低聚物和原纤维模型中研究氟西汀和帕罗西汀的结合相互作用表明,这些SSRI在靠近S形交叉β的N末端(Lys16-Glu22)区域相互作用。链组装并减少Aβ42的原纤维形成。根据这项研究,提出了一种药效团模型,该模型表明设计新型Aβ42聚集抑制剂的最低结构要求包括一个可电离基团,一个疏水基团,两个芳环和两个氢键供体基团的存在。这些研究表明,SSRIs具有通过直接结合防止Aβ42聚集的潜力,并且可能对使用SSRIs的AD患者有益。
更新日期:2018-08-30
中文翻译:

选择性5-羟色胺再摄取抑制剂与β-淀粉样蛋白的相互作用。
目前尚无用于治疗这种破坏性神经退行性疾病的新药,这是治疗阿尔茨海默氏病(AD)的主要挑战。在这方面,旨在确定监管机构已经批准的药物的新颖治疗用途的药物再利用是发现新颖治疗策略的实用方法。选择性5-羟色胺再摄取抑制剂(SSRIs)是美国食品药品监督管理局批准的用于治疗抑郁症的已知药物。我们研究了SSRIs氟伏沙明,氟西汀,帕罗西汀,舍曲林和依他普仑对Aβ42聚集和原纤维形成的能力。值得注意的是,进行的聚集动力学实验表明,在所有测试浓度下,SSRIs氟西汀,帕罗西汀和舍曲林的抗Aβ42聚集活性(1、10,50和100μM)。氟西汀和帕罗西汀均被认为是最有前途的SSRI,在100μM浓度下对Aβ42聚集的抑制率为74.8和76%。透射电子显微镜实验和斑点印迹研究还证明了氟西汀和帕罗西汀具有预防Aβ42聚集和原纤维形成的能力,提供了进一步的证据。在源自固态NMR结构的Aβ42低聚物和原纤维模型中研究氟西汀和帕罗西汀的结合相互作用表明,这些SSRI在靠近S形交叉β的N末端(Lys16-Glu22)区域相互作用。链组装并减少Aβ42的原纤维形成。根据这项研究,提出了一种药效团模型,该模型表明设计新型Aβ42聚集抑制剂的最低结构要求包括一个可电离基团,一个疏水基团,两个芳环和两个氢键供体基团的存在。这些研究表明,SSRIs具有通过直接结合防止Aβ42聚集的潜力,并且可能对使用SSRIs的AD患者有益。











































 京公网安备 11010802027423号
京公网安备 11010802027423号