Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structures of filaments from Pick’s disease reveal a novel tau protein fold
Nature ( IF 50.5 ) Pub Date : 2018-08-29 , DOI: 10.1038/s41586-018-0454-y Benjamin Falcon 1 , Wenjuan Zhang 1 , Alexey G Murzin 1 , Garib Murshudov 1 , Holly J Garringer 2 , Ruben Vidal 2 , R Anthony Crowther 1 , Bernardino Ghetti 2 , Sjors H W Scheres 1 , Michel Goedert 1
Nature ( IF 50.5 ) Pub Date : 2018-08-29 , DOI: 10.1038/s41586-018-0454-y Benjamin Falcon 1 , Wenjuan Zhang 1 , Alexey G Murzin 1 , Garib Murshudov 1 , Holly J Garringer 2 , Ruben Vidal 2 , R Anthony Crowther 1 , Bernardino Ghetti 2 , Sjors H W Scheres 1 , Michel Goedert 1
Affiliation
|
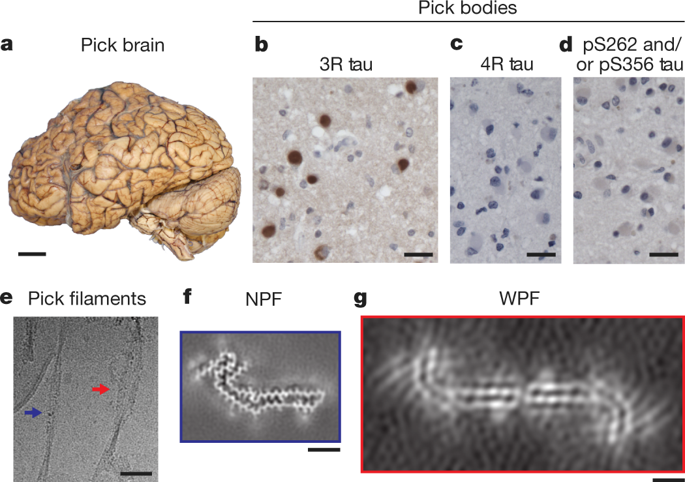
The ordered assembly of tau protein into abnormal filamentous inclusions underlies many human neurodegenerative diseases1. Tau assemblies seem to spread through specific neural networks in each disease2, with short filaments having the greatest seeding activity3. The abundance of tau inclusions strongly correlates with disease symptoms4. Six tau isoforms are expressed in the normal adult human brain—three isoforms with four microtubule-binding repeats each (4R tau) and three isoforms that lack the second repeat (3R tau)1. In various diseases, tau filaments can be composed of either 3R or 4R tau, or of both. Tau filaments have distinct cellular and neuroanatomical distributions5, with morphological and biochemical differences suggesting that they may be able to adopt disease-specific molecular conformations6,7. Such conformers may give rise to different neuropathological phenotypes8,9, reminiscent of prion strains10. However, the underlying structures are not known. Using electron cryo-microscopy, we recently reported the structures of tau filaments from patients with Alzheimer’s disease, which contain both 3R and 4R tau11. Here we determine the structures of tau filaments from patients with Pick’s disease, a neurodegenerative disorder characterized by frontotemporal dementia. The filaments consist of residues Lys254–Phe378 of 3R tau, which are folded differently from the tau filaments in Alzheimer’s disease, establishing the existence of conformers of assembled tau. The observed tau fold in the filaments of patients with Pick’s disease explains the selective incorporation of 3R tau in Pick bodies, and the differences in phosphorylation relative to the tau filaments of Alzheimer’s disease. Our findings show how tau can adopt distinct folds in the human brain in different diseases, an essential step for understanding the formation and propagation of molecular conformers.The structures of tau filaments from patients with the neurodegenerative disorder Pick’s disease show that the filament fold is different from that of the tau filaments found in Alzheimer’s disease.
中文翻译:

皮克氏病的丝状结构揭示了一种新的 tau 蛋白折叠
tau 蛋白有序组装成异常丝状内含物是许多人类神经退行性疾病的基础。 Tau 蛋白组装体似乎通过每种疾病的特定神经网络传播,其中短丝具有最大的播种活性3。 tau 内含物的丰度与疾病症状密切相关4。正常成人大脑中表达六种 tau 异构体,其中三种异构体各有四个微管结合重复序列 (4R tau),三种异构体缺乏第二个重复序列 (3R tau)1。在各种疾病中,tau 蛋白丝可由 3R tau 蛋白或 4R tau 蛋白组成,或由两者组成。 Tau 丝具有独特的细胞和神经解剖学分布 5,形态和生化差异表明它们可能能够采用疾病特异性的分子构象 6,7。这种构象异构体可能会产生不同的神经病理学表型8,9,让人想起朊病毒株10。然而,底层结构尚不清楚。我们最近利用电子冷冻显微镜报道了阿尔茨海默病患者的 tau 丝结构,其中同时含有 3R 和 4R tau11。在这里,我们确定了皮克氏病患者的 tau 蛋白丝结构,皮克氏病是一种以额颞叶痴呆为特征的神经退行性疾病。这些丝由 3R tau 的残基 Lys254–Phe378 组成,其折叠方式与阿尔茨海默病中的 tau 丝不同,证实了组装的 tau 构象异构体的存在。在匹克氏病患者的肌丝中观察到的 tau 蛋白折叠解释了 3R tau 蛋白在匹克氏体中的选择性掺入,以及相对于阿尔茨海默氏病的 tau 蛋白丝的磷酸化差异。 我们的研究结果表明,在不同的疾病中,tau蛋白如何在人脑中形成不同的折叠,这是理解分子构象异构体的形成和传播的重要一步。神经退行性疾病皮克氏病患者的tau蛋白丝结构表明,丝蛋白丝折叠是不同的来自阿尔茨海默病中发现的 tau 蛋白丝。
更新日期:2018-08-29
中文翻译:

皮克氏病的丝状结构揭示了一种新的 tau 蛋白折叠
tau 蛋白有序组装成异常丝状内含物是许多人类神经退行性疾病的基础。 Tau 蛋白组装体似乎通过每种疾病的特定神经网络传播,其中短丝具有最大的播种活性3。 tau 内含物的丰度与疾病症状密切相关4。正常成人大脑中表达六种 tau 异构体,其中三种异构体各有四个微管结合重复序列 (4R tau),三种异构体缺乏第二个重复序列 (3R tau)1。在各种疾病中,tau 蛋白丝可由 3R tau 蛋白或 4R tau 蛋白组成,或由两者组成。 Tau 丝具有独特的细胞和神经解剖学分布 5,形态和生化差异表明它们可能能够采用疾病特异性的分子构象 6,7。这种构象异构体可能会产生不同的神经病理学表型8,9,让人想起朊病毒株10。然而,底层结构尚不清楚。我们最近利用电子冷冻显微镜报道了阿尔茨海默病患者的 tau 丝结构,其中同时含有 3R 和 4R tau11。在这里,我们确定了皮克氏病患者的 tau 蛋白丝结构,皮克氏病是一种以额颞叶痴呆为特征的神经退行性疾病。这些丝由 3R tau 的残基 Lys254–Phe378 组成,其折叠方式与阿尔茨海默病中的 tau 丝不同,证实了组装的 tau 构象异构体的存在。在匹克氏病患者的肌丝中观察到的 tau 蛋白折叠解释了 3R tau 蛋白在匹克氏体中的选择性掺入,以及相对于阿尔茨海默氏病的 tau 蛋白丝的磷酸化差异。 我们的研究结果表明,在不同的疾病中,tau蛋白如何在人脑中形成不同的折叠,这是理解分子构象异构体的形成和传播的重要一步。神经退行性疾病皮克氏病患者的tau蛋白丝结构表明,丝蛋白丝折叠是不同的来自阿尔茨海默病中发现的 tau 蛋白丝。











































 京公网安备 11010802027423号
京公网安备 11010802027423号