Nature Reviews Chemistry ( IF 38.1 ) Pub Date : 2018-08-29 , DOI: 10.1038/s41570-018-0032-8 Arnab Dutta , Aaron M. Appel , Wendy J. Shaw
|
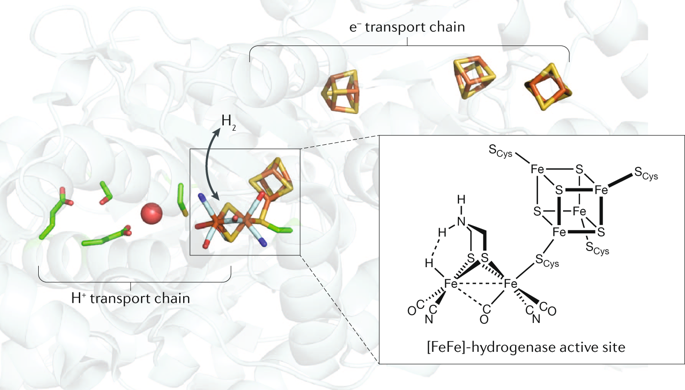
The most energy-efficient electrocatalysts mediate forward and reverse reactions at high rates with minimal overpotential requirements. Such electrocatalytic reversibility is commonly observed for redox enzymes and is an attribute that we have sought to bestow on synthetic molecules to realize highly active and robust catalysts for applications in renewable energy. The recent development of the first synthetic molecular catalysts that reversibly mediate H2 ⇌ 2 H+ + 2e− exploits an enzyme-inspired outer coordination sphere that works in concert with both first and second coordination spheres. In this Perspective, we discuss a series of molecular Ni catalysts for H2 production and oxidation that exhibit electrochemical reversibility. Study of these catalysts allows us to identify important first, second and outer coordination sphere features necessary for efficient conversions of H2 and provides direction for the rational design of electrocatalysts that operate on other small molecules.
中文翻译:

设计电化学可逆的H 2氧化和生产催化剂
最节能的电催化剂可以高速率介导正向和反向反应,而对超电势的要求最小。通常在氧化还原酶中观察到这种电催化可逆性,这是我们试图赋予合成分子以实现可再生能源中应用的高活性和强固性催化剂的一个属性。最近第一合成分子催化剂的发展可逆居间ħ 2 ⇌ 2小时+ + 2e中-利用酶启发外配位球,在音乐会作品与第一和第二配位层。在此观点中,我们讨论了一系列用于H 2的分子Ni催化剂生产和氧化具有电化学可逆性。对这些催化剂的研究使我们能够确定有效转化H 2所必需的重要的第一,第二和外部配位球特征,并为合理设计在其他小分子上运行的电催化剂提供了指导。











































 京公网安备 11010802027423号
京公网安备 11010802027423号