当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Na2S2O8-mediated efficient synthesis of isothiocyanates from primary amines in water
Green Chemistry ( IF 9.3 ) Pub Date : 2018-08-29 , DOI: 10.1039/c8gc02261e Zhicheng Fu 1, 2, 3, 4, 5 , Wenhao Yuan 1, 2, 3, 4, 5 , Ning Chen 1, 2, 3, 4, 5 , Zhanhui Yang 1, 2, 3, 4, 5 , Jiaxi Xu 1, 2, 3, 4, 5
Green Chemistry ( IF 9.3 ) Pub Date : 2018-08-29 , DOI: 10.1039/c8gc02261e Zhicheng Fu 1, 2, 3, 4, 5 , Wenhao Yuan 1, 2, 3, 4, 5 , Ning Chen 1, 2, 3, 4, 5 , Zhanhui Yang 1, 2, 3, 4, 5 , Jiaxi Xu 1, 2, 3, 4, 5
Affiliation
|
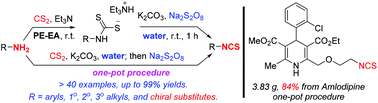
We have developed two green, practical, and efficient procedures, including a one-pot one, to synthesize isothiocyanates from amines and carbon disulfide via desulfurization with sodium persulfate. Water is used as the solvent. Basic conditions are necessary for good chemoselectivity for isothiocyanates. Structurally diverse linear and branched alkyl amines and aryl amines are readily converted to isothiocyanates by the two procedures in satisfactory yields. Halogens, benzylic C–H bonds, methylthio, nitro, ester, alkenyl, electron-rich or -deficient (hetero)aryls, acetylenyl, and even phenolic and alcoholic hydroxyls are well tolerated. The one-pot procedure in water can also be used to realize the preparation of chiral isothiocyanates from chiral amines, and the modification of bioactive structures with free amino groups. In large-scale preparation, simple and practical purification procedures independent of column chromatography are developed.
中文翻译:

Na 2 S 2 O 8介导的水中伯胺高效合成异硫氰酸酯
我们已经开发了两种绿色,实用且有效的方法,包括一锅法,可通过胺和二硫化碳通过胺合成异硫氰酸酯。用过硫酸钠脱硫。使用水作为溶剂。为了使异硫氰酸酯具有良好的化学选择性,必须具备碱性条件。通过两种方法,结构令人满意的直链和支链烷基胺和芳基胺很容易以令人满意的产率转化为异硫氰酸酯。卤素,苄基CH键,甲硫基,硝基,酯,烯基,富电子或不足的(杂)芳基,乙炔基,甚至酚和醇羟基均被很好地耐受。在水中一锅法也可用于从手性胺制备手性异硫氰酸酯,以及用游离氨基修饰生物活性结构。在大规模制备中,开发了独立于柱色谱法的简单实用的纯化方法。
更新日期:2018-10-01
中文翻译:

Na 2 S 2 O 8介导的水中伯胺高效合成异硫氰酸酯
我们已经开发了两种绿色,实用且有效的方法,包括一锅法,可通过胺和二硫化碳通过胺合成异硫氰酸酯。用过硫酸钠脱硫。使用水作为溶剂。为了使异硫氰酸酯具有良好的化学选择性,必须具备碱性条件。通过两种方法,结构令人满意的直链和支链烷基胺和芳基胺很容易以令人满意的产率转化为异硫氰酸酯。卤素,苄基CH键,甲硫基,硝基,酯,烯基,富电子或不足的(杂)芳基,乙炔基,甚至酚和醇羟基均被很好地耐受。在水中一锅法也可用于从手性胺制备手性异硫氰酸酯,以及用游离氨基修饰生物活性结构。在大规模制备中,开发了独立于柱色谱法的简单实用的纯化方法。










































 京公网安备 11010802027423号
京公网安备 11010802027423号