当前位置:
X-MOL 学术
›
Chem. Soc. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Amide activation: an emerging tool for chemoselective synthesis
Chemical Society Reviews ( IF 40.4 ) Pub Date : 2018-08-28 00:00:00 , DOI: 10.1039/c8cs00335a Daniel Kaiser 1 , Adriano Bauer , Miran Lemmerer , Nuno Maulide
Chemical Society Reviews ( IF 40.4 ) Pub Date : 2018-08-28 00:00:00 , DOI: 10.1039/c8cs00335a Daniel Kaiser 1 , Adriano Bauer , Miran Lemmerer , Nuno Maulide
Affiliation
|
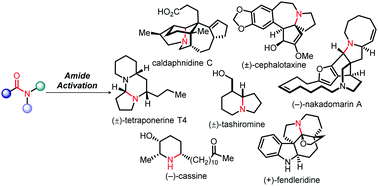
It is textbook knowledge that carboxamides benefit from increased stabilisation of the electrophilic carbonyl carbon when compared to other carbonyl and carboxyl derivatives. This results in a considerably reduced reactivity towards nucleophiles. Accordingly, a perception has been developed of amides as significantly less useful functional handles than their ester and acid chloride counterparts. However, a significant body of research on the selective activation of amides to achieve powerful transformations under mild conditions has emerged over the past decades. This review article aims at placing electrophilic amide activation in both a historical context and in that of natural product synthesis, highlighting the synthetic applications and the potential of this approach.
中文翻译:

酰胺活化:化学选择性合成的新兴工具
与其他羰基和羧基衍生物相比,甲酰胺受益于亲电羰基碳的稳定性增加,这是教科书知识。这导致对亲核试剂的反应性显着降低。因此,人们认为酰胺作为功能性手柄比它们的酯和酰氯对应物显着不那么有用。然而,在过去的几十年中,出现了大量关于选择性激活酰胺以在温和条件下实现强大转化的研究。这篇综述文章旨在将亲电酰胺活化置于历史背景和天然产物合成中,强调合成应用和这种方法的潜力。
更新日期:2018-08-28
中文翻译:

酰胺活化:化学选择性合成的新兴工具
与其他羰基和羧基衍生物相比,甲酰胺受益于亲电羰基碳的稳定性增加,这是教科书知识。这导致对亲核试剂的反应性显着降低。因此,人们认为酰胺作为功能性手柄比它们的酯和酰氯对应物显着不那么有用。然而,在过去的几十年中,出现了大量关于选择性激活酰胺以在温和条件下实现强大转化的研究。这篇综述文章旨在将亲电酰胺活化置于历史背景和天然产物合成中,强调合成应用和这种方法的潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号