当前位置:
X-MOL 学术
›
ACS Cent. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iridium–Tungsten Alloy Nanodendrites as pH-Universal Water-Splitting Electrocatalysts
ACS Central Science ( IF 12.7 ) Pub Date : 2018-08-29 00:00:00 , DOI: 10.1021/acscentsci.8b00426 Fan Lv 1 , Jianrui Feng 1 , Kai Wang 1 , Zhipeng Dou 2 , Weiyu Zhang 1 , Jinhui Zhou 1 , Chao Yang 1 , Mingchuan Luo 1 , Yong Yang 1 , Yingjie Li 1 , Peng Gao 2 , Shaojun Guo 1, 3, 4, 5
ACS Central Science ( IF 12.7 ) Pub Date : 2018-08-29 00:00:00 , DOI: 10.1021/acscentsci.8b00426 Fan Lv 1 , Jianrui Feng 1 , Kai Wang 1 , Zhipeng Dou 2 , Weiyu Zhang 1 , Jinhui Zhou 1 , Chao Yang 1 , Mingchuan Luo 1 , Yong Yang 1 , Yingjie Li 1 , Peng Gao 2 , Shaojun Guo 1, 3, 4, 5
Affiliation
|
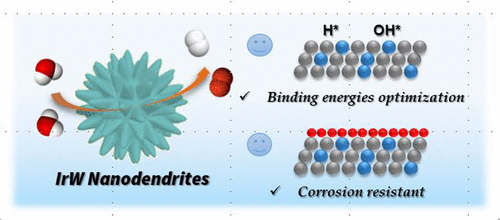
The development of highly efficient and durable electrocatalysts for high-performance overall water-splitting devices is crucial for clean energy conversion. However, the existing electrocatalysts still suffer from low catalytic efficiency, and need a large overpotential to drive the overall water-splitting reactions. Herein, we report an iridium–tungsten alloy with nanodendritic structure (IrW ND) as a new class of high-performance and pH-universal bifunctional electrocatalysts for hydrogen and oxygen evolution catalysis. The IrW ND catalyst presents a hydrogen generation rate ∼2 times higher than that of the commercial Pt/C catalyst in both acid and alkaline media, which is among the most active hydrogen evolution reaction (HER) catalysts yet reported. The density functional theory (DFT) calculations reveal that the high HER intrinsic catalytic activity results from the suitable hydrogen and hydroxyl binding energies, which can accelerate the rate-determining step of the HER in acid and alkaline media. Moreover, the IrW NDs show superb oxygen evolution reaction (OER) activity and much improved stability over Ir. The theoretical calculation demonstrates that alloying Ir metal with W can stabilize the formed active iridium oxide during the OER process and lower the binding energy of reaction intermediates, thus improving the Ir corrosion resistance and OER kinetics. Furthermore, the overall water-splitting devices driven by IrW NDs can work in a wide pH range and achieve a current density of 10 mA cm–2 in acid electrolyte at a low potential of 1.48 V.
中文翻译:

铱-钨合金纳米枝晶作为pH值通用的水分解电催化剂
用于高性能整体水分解装置的高效耐用的电催化剂的开发对于清洁能源的转化至关重要。然而,现有的电催化剂仍然遭受催化效率低的问题,并且需要大的超电势来驱动整个水分解反应。在本文中,我们报告了具有纳米树枝状结构的铱钨合金(IrW ND),它是一类新型的可在氢和氧逸出催化中使用的高性能且具有pH值通用性的双功能电催化剂。IrW ND催化剂在酸性和碱性介质中的产氢率均比市售Pt / C催化剂高约2倍,这是迄今报道的活性最高的析氢反应(HER)催化剂之一。密度泛函理论(DFT)计算表明,较高的HER固有催化活性是由合适的氢和羟基结合能产生的,这可以加快HER在酸和碱性介质中的速率确定步骤。此外,IrW NDs表现出了出色的氧释放反应(OER)活性,并且与Ir相比,稳定性大大提高。理论计算表明,将Ir金属与W合金化可以稳定OER过程中形成的活性氧化铱,降低反应中间体的结合能,从而提高Ir的耐蚀性和OER动力学。此外,由IrW ND驱动的整个水分解装置可在较宽的pH范围内工作,并实现10 mA cm的电流密度 这可以加快在酸性和碱性介质中HER的速率测定步骤。此外,IrW NDs表现出了出色的氧释放反应(OER)活性,并且与Ir相比,稳定性大大提高。理论计算表明,将Ir金属与W合金化可以稳定OER过程中形成的活性氧化铱,降低反应中间体的结合能,从而提高Ir的耐蚀性和OER动力学。此外,由IrW ND驱动的整个水分解装置可在较宽的pH范围内工作,并实现10 mA cm的电流密度 这可以加快在酸性和碱性介质中HER的速率测定步骤。此外,IrW NDs表现出了出色的氧释放反应(OER)活性,并且与Ir相比,稳定性大大提高。理论计算表明,将Ir金属与W合金化可以稳定OER过程中形成的活性氧化铱,降低反应中间体的结合能,从而提高Ir的耐蚀性和OER动力学。此外,由IrW ND驱动的整个水分解装置可在较宽的pH范围内工作,并实现10 mA cm的电流密度 理论计算表明,将Ir金属与W合金化可以稳定OER过程中形成的活性氧化铱,降低反应中间体的结合能,从而提高Ir的耐蚀性和OER动力学。此外,由IrW ND驱动的整个水分解装置可在较宽的pH范围内工作,并实现10 mA cm的电流密度 理论计算表明,将Ir金属与W合金化可以稳定OER过程中形成的活性氧化铱,降低反应中间体的结合能,从而提高Ir的耐蚀性和OER动力学。此外,由IrW ND驱动的整个水分解装置可在较宽的pH范围内工作,并实现10 mA cm的电流密度酸性电解液中的–2在1.48 V的低电势下。
更新日期:2018-08-29
中文翻译:

铱-钨合金纳米枝晶作为pH值通用的水分解电催化剂
用于高性能整体水分解装置的高效耐用的电催化剂的开发对于清洁能源的转化至关重要。然而,现有的电催化剂仍然遭受催化效率低的问题,并且需要大的超电势来驱动整个水分解反应。在本文中,我们报告了具有纳米树枝状结构的铱钨合金(IrW ND),它是一类新型的可在氢和氧逸出催化中使用的高性能且具有pH值通用性的双功能电催化剂。IrW ND催化剂在酸性和碱性介质中的产氢率均比市售Pt / C催化剂高约2倍,这是迄今报道的活性最高的析氢反应(HER)催化剂之一。密度泛函理论(DFT)计算表明,较高的HER固有催化活性是由合适的氢和羟基结合能产生的,这可以加快HER在酸和碱性介质中的速率确定步骤。此外,IrW NDs表现出了出色的氧释放反应(OER)活性,并且与Ir相比,稳定性大大提高。理论计算表明,将Ir金属与W合金化可以稳定OER过程中形成的活性氧化铱,降低反应中间体的结合能,从而提高Ir的耐蚀性和OER动力学。此外,由IrW ND驱动的整个水分解装置可在较宽的pH范围内工作,并实现10 mA cm的电流密度 这可以加快在酸性和碱性介质中HER的速率测定步骤。此外,IrW NDs表现出了出色的氧释放反应(OER)活性,并且与Ir相比,稳定性大大提高。理论计算表明,将Ir金属与W合金化可以稳定OER过程中形成的活性氧化铱,降低反应中间体的结合能,从而提高Ir的耐蚀性和OER动力学。此外,由IrW ND驱动的整个水分解装置可在较宽的pH范围内工作,并实现10 mA cm的电流密度 这可以加快在酸性和碱性介质中HER的速率测定步骤。此外,IrW NDs表现出了出色的氧释放反应(OER)活性,并且与Ir相比,稳定性大大提高。理论计算表明,将Ir金属与W合金化可以稳定OER过程中形成的活性氧化铱,降低反应中间体的结合能,从而提高Ir的耐蚀性和OER动力学。此外,由IrW ND驱动的整个水分解装置可在较宽的pH范围内工作,并实现10 mA cm的电流密度 理论计算表明,将Ir金属与W合金化可以稳定OER过程中形成的活性氧化铱,降低反应中间体的结合能,从而提高Ir的耐蚀性和OER动力学。此外,由IrW ND驱动的整个水分解装置可在较宽的pH范围内工作,并实现10 mA cm的电流密度 理论计算表明,将Ir金属与W合金化可以稳定OER过程中形成的活性氧化铱,降低反应中间体的结合能,从而提高Ir的耐蚀性和OER动力学。此外,由IrW ND驱动的整个水分解装置可在较宽的pH范围内工作,并实现10 mA cm的电流密度酸性电解液中的–2在1.48 V的低电势下。











































 京公网安备 11010802027423号
京公网安备 11010802027423号