当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermochemically Consistent Free Energies of Hydration for Di- and Trivalent Metal Ions
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-08-29 00:00:00 , DOI: 10.1021/acs.jpca.8b06674 Kasper P. Kepp 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-08-29 00:00:00 , DOI: 10.1021/acs.jpca.8b06674 Kasper P. Kepp 1
Affiliation
|
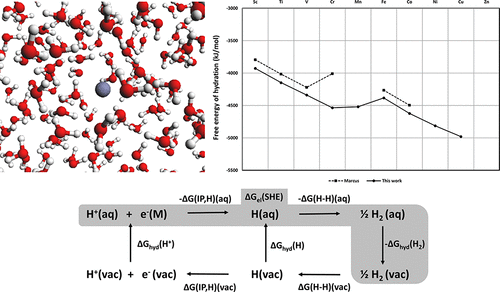
This paper uses the relationship between the standard half reduction potential, the third ionization potential, and the free energies of hydration (ΔGhyd) of M2+ and M3+ ions to calculate new values of ΔGhyd for M2+ and M3+ ions. The numbers are “thermochemically consistent”; i.e., all numbers agree with the applied thermochemical cycle. This enables the tabulation of many ΔGhyd derived mainly from the data compiled by Marcus, but consistent with ΔGhyd(H+) = 1100 kJ/mol and SHE = 4.44 V. The accuracy of the new values of ΔGhyd(M3+) is by definition similar to the accuracy of the experimental hydration energy of the ΔGhyd(M2+) used for calculation, and vice versa, i.e. the new data have the same accuracy or higher than previously reported. As a result, the literature values for Cr3+ and Au3+, and Pd2+ are substantially revised. The approach also allows the calculation of new ΔGhyd for metal ions such as Mn3+, Ti2+, Ag3+, Ni3+, Cu3+, and Au2+ and the theoretically interesting but experimentally inaccessible +2 ions of lanthanides. The new numbers enable a discussion of the previously unreported trend in ΔGhyd(M3+) for the 3d metal ions, which relates to the ligand field stabilization energies and effective nuclear charge as for the M2+ ions. The new tabulated values should be accurate with the applied assumptions to within 10 kJ/mol and may be of value for other thermochemical calculations, for interpretation of the aqueous trend chemistry of the metal ions, and as benchmarks for theoretical chemistry.
中文翻译:

二价和三价金属离子水合的热化学一致自由能
本文使用标准的半还原电势之间的第三电离电势和水合(Δ的自由能的关系,ģ水合M的)2+和M 3+离子,计算Δ的新值G ^ HYD对于M 2+和M 3+离子。这些数字是“热化学一致的”;即,所有数字均与所应用的热化学循环一致。这使得许多Δ的制表ģ HYD主要来源于由Marcus编译的数据,而是符合Δ ģ水合(H +)= 1100千焦/摩尔和SHE = 4.44 V.Δ的新值的准确性ģ水合根据定义,(M 3+)类似于用于计算的ΔG hyd(M 2+)的实验水合能的精度,反之亦然,即,新数据具有相同的精度或比以前报告的精度更高。结果,对Cr 3+和Au 3+以及Pd 2+的文献值进行了实质性修改。该方法还允许计算金属离子(例如Mn 3+,Ti 2 +,Ag 3+,Ni 3+,Cu 3+和Au 2+)的新ΔG hyd和理论上有趣但实验上无法接近的镧系离子+2离子。新的数字可以讨论3d金属离子的ΔG hyd(M 3+)以前未报告的趋势,该趋势与M 2+离子的配体场稳定能和有效核电荷有关。在适用的假设下,新的列表值应准确,且应在10 kJ / mol以内,并可能对其他热化学计算,解释金属离子的水态趋势化学以及作为理论化学的基准具有参考价值。
更新日期:2018-08-29
中文翻译:

二价和三价金属离子水合的热化学一致自由能
本文使用标准的半还原电势之间的第三电离电势和水合(Δ的自由能的关系,ģ水合M的)2+和M 3+离子,计算Δ的新值G ^ HYD对于M 2+和M 3+离子。这些数字是“热化学一致的”;即,所有数字均与所应用的热化学循环一致。这使得许多Δ的制表ģ HYD主要来源于由Marcus编译的数据,而是符合Δ ģ水合(H +)= 1100千焦/摩尔和SHE = 4.44 V.Δ的新值的准确性ģ水合根据定义,(M 3+)类似于用于计算的ΔG hyd(M 2+)的实验水合能的精度,反之亦然,即,新数据具有相同的精度或比以前报告的精度更高。结果,对Cr 3+和Au 3+以及Pd 2+的文献值进行了实质性修改。该方法还允许计算金属离子(例如Mn 3+,Ti 2 +,Ag 3+,Ni 3+,Cu 3+和Au 2+)的新ΔG hyd和理论上有趣但实验上无法接近的镧系离子+2离子。新的数字可以讨论3d金属离子的ΔG hyd(M 3+)以前未报告的趋势,该趋势与M 2+离子的配体场稳定能和有效核电荷有关。在适用的假设下,新的列表值应准确,且应在10 kJ / mol以内,并可能对其他热化学计算,解释金属离子的水态趋势化学以及作为理论化学的基准具有参考价值。











































 京公网安备 11010802027423号
京公网安备 11010802027423号