当前位置:
X-MOL 学术
›
ACS Cent. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Free Energy Landscape of the Complete Transport Cycle in a Key Bacterial Transporter
ACS Central Science ( IF 12.7 ) Pub Date : 2018-08-28 00:00:00 , DOI: 10.1021/acscentsci.8b00330 Balaji Selvam , Shriyaa Mittal , Diwakar Shukla
ACS Central Science ( IF 12.7 ) Pub Date : 2018-08-28 00:00:00 , DOI: 10.1021/acscentsci.8b00330 Balaji Selvam , Shriyaa Mittal , Diwakar Shukla
|
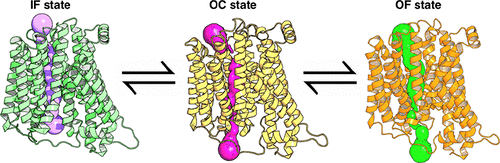
PepTSo is a proton-coupled bacterial symporter, from the major facilitator superfamily (MFS), which transports di-/tripeptide molecules. The recently obtained crystal structure of PepTSo provides an unprecedented opportunity to gain an understanding of functional insights of the substrate transport mechanism. Binding of the proton and peptide molecule induces conformational changes into occluded (OC) and outward-facing (OF) states, which we are able to characterize using molecular dynamics (MD) simulations. The structural knowledge of the OC and OF state is important to fully understand the major energy barrier associated with the transport cycle. In order to gain functional insight into the interstate dynamics, we performed extensive all atom MD simulations. The Markov state model was constructed to identify the free energy barriers between the states, and kinetic information on intermediate pathways was obtained using the transition pathway theory (TPT). TPT shows that the OF state is obtained by the movement of TM1 and TM7 at the extracellular side approximately 12–16 Å away from each other, and the inward movement of TM4 and TM10 at the intracellular halves to 3–4 Å characterizes the OC state. Helix distance distributions obtained from MD simulations were compared with experimental double electron–electron resonance spectroscopy and were found to be in excellent agreement with previous studies. We also predicted the optimal positions for placement of methane thiosulfonate spin label probes to capture the slowest protein dynamics. Our finding sheds light on the conformational cycle of this key membrane transporter and the functional relationships between the multiple intermediate states.
中文翻译:

关键细菌转运蛋白中完整运输周期的自由能态势
PepT So是质子耦合的细菌同向转运蛋白,来自主要的辅助超家族(MFS),可转运二肽/三肽分子。最近获得的PepT So的晶体结构提供了前所未有的机会来了解基材传输机制的功能见解。质子和肽分子的结合诱导构象变化为闭塞(OC)和朝外(OF)状态,我们可以使用分子动力学(MD)模拟来表征。OC和OF状态的结构知识对于充分了解与运输周期相关的主要能量壁垒非常重要。为了获得对州际动力学的功能见解,我们进行了广泛的所有原子MD模拟。建立马尔可夫状态模型以识别状态之间的自由能垒,并使用过渡路径理论(TPT)获得有关中间路径的动力学信息。TPT显示OF状态是通过TM1和TM7在细胞外侧彼此之间约12-16Å的移动获得的,而TM4和TM10在细胞内半部向3-4Å的向内移动是OC状态的特征。通过MD模拟获得的螺旋距离分布与实验性双电子-电子共振光谱进行了比较,发现与先前的研究非常吻合。我们还预测了放置甲烷硫代磺酸盐自旋标记探针的最佳位置,以捕获最慢的蛋白质动力学。我们的发现揭示了该关键膜转运蛋白的构象循环以及多个中间状态之间的功能关系。而TM4和TM10在细胞内半部向内移动至3-4Å则表明OC状态。通过MD模拟获得的螺旋距离分布与实验性双电子-电子共振光谱进行了比较,发现与先前的研究非常吻合。我们还预测了放置甲烷硫代磺酸盐自旋标记探针的最佳位置,以捕获最慢的蛋白质动力学。我们的发现揭示了该关键膜转运蛋白的构象循环以及多个中间状态之间的功能关系。而TM4和TM10在细胞内半部向内移动至3-4Å则表明OC状态。通过MD模拟获得的螺旋距离分布与实验性双电子-电子共振光谱进行了比较,发现与先前的研究非常吻合。我们还预测了放置甲烷硫代磺酸盐自旋标记探针的最佳位置,以捕获最慢的蛋白质动力学。我们的发现揭示了该关键膜转运蛋白的构象循环以及多个中间状态之间的功能关系。通过MD模拟获得的螺旋距离分布与实验性双电子-电子共振光谱进行了比较,发现与先前的研究非常吻合。我们还预测了放置甲烷硫代磺酸盐自旋标记探针的最佳位置,以捕获最慢的蛋白质动力学。我们的发现揭示了该关键膜转运蛋白的构象循环以及多个中间状态之间的功能关系。通过MD模拟获得的螺旋距离分布与实验性双电子-电子共振光谱进行了比较,发现与先前的研究非常吻合。我们还预测了放置甲烷硫代磺酸盐自旋标记探针的最佳位置,以捕获最慢的蛋白质动力学。我们的发现揭示了该关键膜转运蛋白的构象循环以及多个中间状态之间的功能关系。
更新日期:2018-08-28
中文翻译:

关键细菌转运蛋白中完整运输周期的自由能态势
PepT So是质子耦合的细菌同向转运蛋白,来自主要的辅助超家族(MFS),可转运二肽/三肽分子。最近获得的PepT So的晶体结构提供了前所未有的机会来了解基材传输机制的功能见解。质子和肽分子的结合诱导构象变化为闭塞(OC)和朝外(OF)状态,我们可以使用分子动力学(MD)模拟来表征。OC和OF状态的结构知识对于充分了解与运输周期相关的主要能量壁垒非常重要。为了获得对州际动力学的功能见解,我们进行了广泛的所有原子MD模拟。建立马尔可夫状态模型以识别状态之间的自由能垒,并使用过渡路径理论(TPT)获得有关中间路径的动力学信息。TPT显示OF状态是通过TM1和TM7在细胞外侧彼此之间约12-16Å的移动获得的,而TM4和TM10在细胞内半部向3-4Å的向内移动是OC状态的特征。通过MD模拟获得的螺旋距离分布与实验性双电子-电子共振光谱进行了比较,发现与先前的研究非常吻合。我们还预测了放置甲烷硫代磺酸盐自旋标记探针的最佳位置,以捕获最慢的蛋白质动力学。我们的发现揭示了该关键膜转运蛋白的构象循环以及多个中间状态之间的功能关系。而TM4和TM10在细胞内半部向内移动至3-4Å则表明OC状态。通过MD模拟获得的螺旋距离分布与实验性双电子-电子共振光谱进行了比较,发现与先前的研究非常吻合。我们还预测了放置甲烷硫代磺酸盐自旋标记探针的最佳位置,以捕获最慢的蛋白质动力学。我们的发现揭示了该关键膜转运蛋白的构象循环以及多个中间状态之间的功能关系。而TM4和TM10在细胞内半部向内移动至3-4Å则表明OC状态。通过MD模拟获得的螺旋距离分布与实验性双电子-电子共振光谱进行了比较,发现与先前的研究非常吻合。我们还预测了放置甲烷硫代磺酸盐自旋标记探针的最佳位置,以捕获最慢的蛋白质动力学。我们的发现揭示了该关键膜转运蛋白的构象循环以及多个中间状态之间的功能关系。通过MD模拟获得的螺旋距离分布与实验性双电子-电子共振光谱进行了比较,发现与先前的研究非常吻合。我们还预测了放置甲烷硫代磺酸盐自旋标记探针的最佳位置,以捕获最慢的蛋白质动力学。我们的发现揭示了该关键膜转运蛋白的构象循环以及多个中间状态之间的功能关系。通过MD模拟获得的螺旋距离分布与实验性双电子-电子共振光谱进行了比较,发现与先前的研究非常吻合。我们还预测了放置甲烷硫代磺酸盐自旋标记探针的最佳位置,以捕获最慢的蛋白质动力学。我们的发现揭示了该关键膜转运蛋白的构象循环以及多个中间状态之间的功能关系。









































 京公网安备 11010802027423号
京公网安备 11010802027423号