Bioorganic Chemistry ( IF 5.1 ) Pub Date : 2018-08-28 , DOI: 10.1016/j.bioorg.2018.08.035 Shrinivas D. Joshi , Sheshagiri R. Dixit , Jeelan Basha , V.H. Kulkarni , Tejraj M. Aminabhavi , Mallikarjuna N. Nadagouda , Christian Lherbet
|
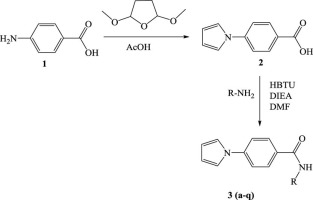
In an effort to produce new lead antimycobacterial compounds, herein we have reported the synthesis of a sequence of new pyrrolyl benzamide derivatives. The new chemical entities were screened to target enoyl-ACP reductase enzyme, which is one of the key enzymes of M. tuberculosis that are involved in type II fatty acid biosynthetic pathway. Compound 3q exhibited H-bonding interactions with Tyr158, Thr196 and co-factor NAD+ that binds the active site of InhA. All the pyrrolyl benzamide compounds were evaluated as inhibitors of M. tuberculosis H37Rv as well as inhibitors of InhA. Among them, few representative compounds were tested for mammalian cell toxicity on the human lung cancer cell-line (A549) and MV cell line that presented no cytotoxicity. Five of these compounds exhibited a good activity against InhA.
中文翻译:

某些新型吡咯基苯甲酰胺衍生物的药理学定位,分子对接,化学合成及其对烯酰基-ACP还原酶(InhA)和结核分枝杆菌的抑制活性
为了生产新的抗分枝杆菌先导化合物,本文中我们报道了新的吡咯基苯甲酰胺衍生物序列的合成。筛选了新的化学实体以靶向烯酰基-ACP还原酶,该酶是参与II型脂肪酸生物合成途径的结核分枝杆菌的关键酶之一。化合物3q与Tyr158,Thr196和与InhA活性位点结合的辅因子NAD +表现出H键相互作用。所有吡咯基苯甲酰胺化合物均被评估为结核分枝杆菌H 37的抑制剂Rv和InhA抑制剂。其中,很少有代表性的化合物对未表现出细胞毒性的人肺癌细胞系(A549)和MV细胞系进行哺乳动物细胞毒性测试。这些化合物中的五个对InhA表现出良好的活性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号