当前位置:
X-MOL 学术
›
ChemElectroChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Competitive Routes for Electrochemical Oxidation of Substituted Diarylamines: the Guidelines
ChemElectroChem ( IF 3.5 ) Pub Date : 2018-09-21 , DOI: 10.1002/celc.201801177 Oleg A. Levitskiy 1 , Dmitry A. Dulov 1 , Oleg M. Nikitin 1 , Alexey V. Bogdanov 1 , Dmitry B. Eremin 2 , Ksenia A. Paseshnichenko 1 , Tatiana V. Magdesieva 1
ChemElectroChem ( IF 3.5 ) Pub Date : 2018-09-21 , DOI: 10.1002/celc.201801177 Oleg A. Levitskiy 1 , Dmitry A. Dulov 1 , Oleg M. Nikitin 1 , Alexey V. Bogdanov 1 , Dmitry B. Eremin 2 , Ksenia A. Paseshnichenko 1 , Tatiana V. Magdesieva 1
Affiliation
|
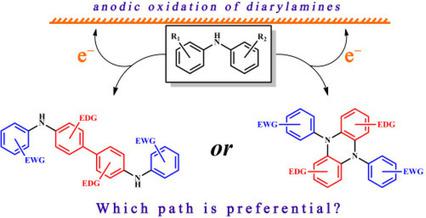
Electrochemical oxidation of diphenylamines with electron‐donating and electron‐withdrawing substituents in various combinations was investigated. It was shown that the subsequent reaction channels for the radical cations are dependent on the location and electronic properties of the substituents in both phenyl rings. Guidelines for the prediction of the dominant reaction path were formulated. The conclusions developed will be useful for planning electrosynthesis. Digital simulation of the voltammograms allowed estimating the mechanism of N,N‐diaryl‐5,10‐dihydrophenazine formation (which is one of the main reaction channels); the corresponding radical cations were isolated for the first time and characterized by X‐ray, electrochemical and spectral methods. Oxidation peak potentials for diarylaminyl anions (obtained using electrochemically generated base) were measured providing information for mechanistic estimation of anti/prooxidant activity of diarylamines in radical processes.
中文翻译:

取代二芳基胺电化学氧化的竞争途径:指南
研究了带有给电子和吸电子取代基的二苯胺在各种组合中的电化学氧化。结果表明,随后的自由基阳离子反应通道取决于两个苯环中取代基的位置和电子性质。制定了预测主要反应路径的指南。得出的结论将对计划电合成有用。伏安图的数字模拟可以估算N,N-二芳基-5,10-二氢吩嗪的形成机理(这是主要的反应通道之一);首次分离了相应的自由基阳离子,并通过X射线,电化学和光谱方法对其进行了表征。
更新日期:2018-09-21
中文翻译:

取代二芳基胺电化学氧化的竞争途径:指南
研究了带有给电子和吸电子取代基的二苯胺在各种组合中的电化学氧化。结果表明,随后的自由基阳离子反应通道取决于两个苯环中取代基的位置和电子性质。制定了预测主要反应路径的指南。得出的结论将对计划电合成有用。伏安图的数字模拟可以估算N,N-二芳基-5,10-二氢吩嗪的形成机理(这是主要的反应通道之一);首次分离了相应的自由基阳离子,并通过X射线,电化学和光谱方法对其进行了表征。











































 京公网安备 11010802027423号
京公网安备 11010802027423号