当前位置:
X-MOL 学术
›
ChemElectroChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Influence of Gd Doping on the Structure and Electrocatalytic Performance of TiO2 Nanotube/SnO2−Sb Nano‐coated Electrode
ChemElectroChem ( IF 3.5 ) Pub Date : 2018-09-21 , DOI: 10.1002/celc.201801079 Lisha Yang 1 , Zhaohan Zhang 1 , Junfeng Liu 1 , Linlin Huang 1 , Liu Jia 1 , Yujie Feng 1
ChemElectroChem ( IF 3.5 ) Pub Date : 2018-09-21 , DOI: 10.1002/celc.201801079 Lisha Yang 1 , Zhaohan Zhang 1 , Junfeng Liu 1 , Linlin Huang 1 , Liu Jia 1 , Yujie Feng 1
Affiliation
|
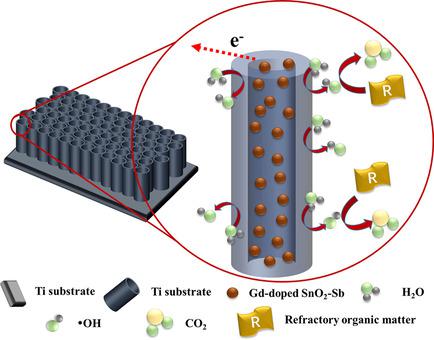
Gd‐doped TiO2−NT/SnO2−Sb (NT=nanotube) electrodes were prepared by using a solvothermal synthesis approach with a nano‐sized catalyst coating. Phenol degradation and total organic carbon (TOC) removal followed pseudo‐first‐order kinetics in the experimental range. A maximum rate was achieved by using a Gd doping ratio of 2 % (molar ratio based on Gd/Sn), which was 56.5 % and 68 % higher than that of the control (Gd/0 %) for phenol degradation and TOC removal. The results from the UV scan of the electrolyte showed that introducing an appropriate amount of Gd could promote the electrochemical incineration process, and thus effectively degrade the chemical intermediates during phenol oxidation. In addition, the Gd/2 %‐doped electrode had the longest accelerated life time of 25 h, which was 25 % higher than that of the control. A suitable Gd doping ratio could diminish the SnO2 crystal size and increase the specific surface area, speeding up the electrode's reaction rate, thus promoting the oxygen evolution potential. A regular and compact morphology with a smallest particle size of 9.5 nm was obtained on the Gd/2 %‐doped electrode, which prompted a smaller charge‐transfer resistance and higher electrical double‐layer capacitance than that of the control. The results from X‐ray photoelectron spectroscopy and electron paramagnetic resonance suggested that a maximal of surface active sites (i. e. oxygen vacancy) was formed on the Gd/2 %‐doped electrode, which provided abundant positive charge for adsorbing more oxygen species (37.5 %) than the control (21.5 %), and greatly enhanced the formation of ·OH to attack the targeted pollutant.
中文翻译:

Gd掺杂对TiO2纳米管/ SnO2-Sb纳米涂层电极结构和电催化性能的影响
掺Gd的TiO 2 -NT / SnO 2-Sb(NT = nanotube)电极是采用溶剂热合成方法制备的,具有纳米级催化剂涂层。在实验范围内,苯酚降解和总有机碳(TOC)去除遵循拟一级动力学。通过使用2%的Gd掺杂率(基于Gd / Sn的摩尔比)实现了最高比率,这比苯酚降解和TOC去除的对照(Gd / 0%)高出56.5%和68%。电解液的UV扫描结果表明,引入适量的Gd可以促进电化学焚烧过程,从而有效地降解苯酚氧化过程中的化学中间体。此外,Gd / 2%掺杂电极具有最长的25 h加速寿命,比对照组高25%。2.增加晶体尺寸并增加比表面积,加快电极的反应速度,从而促进氧的放出电位。在Gd / 2%掺杂的电极上获得了规则且紧凑的形态,最小粒径为9.5 nm,这提示与对照组相比,电荷转移电阻较小,双层电容量较高。X射线光电子能谱和电子顺磁共振的结果表明,在掺杂Gd / 2%的电极上形成了最大的表面活性位(即氧空位),为吸附更多的氧提供了充足的正电荷(37.5%)。 )(21.5%),大大提高了·OH的形成以攻击目标污染物。
更新日期:2018-09-21
中文翻译:

Gd掺杂对TiO2纳米管/ SnO2-Sb纳米涂层电极结构和电催化性能的影响
掺Gd的TiO 2 -NT / SnO 2-Sb(NT = nanotube)电极是采用溶剂热合成方法制备的,具有纳米级催化剂涂层。在实验范围内,苯酚降解和总有机碳(TOC)去除遵循拟一级动力学。通过使用2%的Gd掺杂率(基于Gd / Sn的摩尔比)实现了最高比率,这比苯酚降解和TOC去除的对照(Gd / 0%)高出56.5%和68%。电解液的UV扫描结果表明,引入适量的Gd可以促进电化学焚烧过程,从而有效地降解苯酚氧化过程中的化学中间体。此外,Gd / 2%掺杂电极具有最长的25 h加速寿命,比对照组高25%。2.增加晶体尺寸并增加比表面积,加快电极的反应速度,从而促进氧的放出电位。在Gd / 2%掺杂的电极上获得了规则且紧凑的形态,最小粒径为9.5 nm,这提示与对照组相比,电荷转移电阻较小,双层电容量较高。X射线光电子能谱和电子顺磁共振的结果表明,在掺杂Gd / 2%的电极上形成了最大的表面活性位(即氧空位),为吸附更多的氧提供了充足的正电荷(37.5%)。 )(21.5%),大大提高了·OH的形成以攻击目标污染物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号