当前位置:
X-MOL 学术
›
ChemElectroChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Performance and In‐Situ FTIR Evaluation of Pt−Sn/C Electrocatalysts with Several Pt : Sn Atomic Ratios for the Ethanol Oxidation Reaction in Acidic Media
ChemElectroChem ( IF 4 ) Pub Date : 2018-09-13 , DOI: 10.1002/celc.201800828 D. González-Quijano 1 , W. J. Pech-Rodríguez 2 , J. A. González-Quijano 3 , J. I. Escalante-García 4, 5 , C. Morais 6 , T. W. Napporn 6 , F. J. Rodríguez-Varela 5
ChemElectroChem ( IF 4 ) Pub Date : 2018-09-13 , DOI: 10.1002/celc.201800828 D. González-Quijano 1 , W. J. Pech-Rodríguez 2 , J. A. González-Quijano 3 , J. I. Escalante-García 4, 5 , C. Morais 6 , T. W. Napporn 6 , F. J. Rodríguez-Varela 5
Affiliation
|
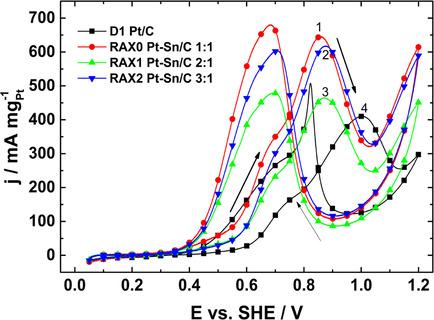
Pt−Sn/C electrocatalysts with nominal Pt : Sn atomic ratios of 1 : 1, 2 : 1 and 3 : 1 were synthesized via the polyol method. The effects of the atomic ratios on their catalytic activity for the ethanol oxidation reaction (EOR) in acidic media was evaluated and compared to a Pt/C electrocatalyst obtained by the same procedure. The differences in reaction mechanism between the alloys and Pt/C were elucidated by in‐situ FTIR measurements. XRD characterization of the Pt−Sn/C electrocatalysts showed a degree of alloying ranging between 27 and 54 % (from Vegard's law), while crystallite sizes close to 2.2 nm were determined with the Scherrer equation. Pt : Sn atomic ratios of 1 : 1, 1.6 : 1 and 2.4 : 1 were found experimentally via EDS analysis, fairly close to the nominally expected values. Electrochemical evaluation indicated that the alloy having a 1 : 1 ratio has the highest electrocatalytic activity for EOR. In‐situ FTIR characterization showed that the Pt−Sn/C alloys promote the oxidation of ethanol via a triple parallel pathway: one path involving the directly conversion of COL (linearly‐bonded CO) to CO2, while the second proceeds via the reaction of AAL (acetaldehyde) into COL and later to CO2, and the third involves the formation of AA (acetic acid) from adsorbed acetate that may be produced from AAL. Such mechanism promoted the EOR at more negative potentials at the alloys, also delivering higher current densities, relative to Pt/C. Thus, the inclusion of Sn in the Pt structure enhanced the catalytic activity for the EOR in acidic media.
中文翻译:

具有多个Pt:Sn原子比的Pt-Sn / C电催化剂在酸性介质中进行乙醇氧化反应的性能和原位FTIR评估
通过多元醇法合成了标称Pt:Sn原子比为1:1、2:1和3:1的Pt-Sn / C电催化剂。评估了原子比对其在酸性介质中对乙醇氧化反应(EOR)的催化活性的影响,并将其与通过相同程序获得的Pt / C电催化剂进行了比较。通过原位FTIR测量阐明了合金与Pt / C之间反应机理的差异。Pt-Sn / C电催化剂的XRD表征表明合金化程度在27%至54%之间(根据维格德定律),而通过Scherrer方程确定了接近2.2 nm的微晶尺寸。通过EDS分析实验发现Pt:Sn原子比为1:1、1.6:1和2.4:1,非常接近标称预期值。电化学评估表明,比例为1:1的合金对EOR的电催化活性最高。原位FTIR表征表明Pt-Sn / C合金通过三重平行途径促进乙醇氧化:一种途径涉及CO的直接转化L(线性键合的CO)转化为CO 2,而第二步通过AAL(乙醛)转化为CO L,然后再转化为CO 2进行,第三步通过吸附的乙酸盐形成AA(乙酸),可能是由AAL生产。相对于Pt / C,这种机制在合金的负电位更高时促进了EOR,还提供了更高的电流密度。因此,在Pt结构中包含Sn增强了在酸性介质中对EOR的催化活性。
更新日期:2018-09-13
中文翻译:

具有多个Pt:Sn原子比的Pt-Sn / C电催化剂在酸性介质中进行乙醇氧化反应的性能和原位FTIR评估
通过多元醇法合成了标称Pt:Sn原子比为1:1、2:1和3:1的Pt-Sn / C电催化剂。评估了原子比对其在酸性介质中对乙醇氧化反应(EOR)的催化活性的影响,并将其与通过相同程序获得的Pt / C电催化剂进行了比较。通过原位FTIR测量阐明了合金与Pt / C之间反应机理的差异。Pt-Sn / C电催化剂的XRD表征表明合金化程度在27%至54%之间(根据维格德定律),而通过Scherrer方程确定了接近2.2 nm的微晶尺寸。通过EDS分析实验发现Pt:Sn原子比为1:1、1.6:1和2.4:1,非常接近标称预期值。电化学评估表明,比例为1:1的合金对EOR的电催化活性最高。原位FTIR表征表明Pt-Sn / C合金通过三重平行途径促进乙醇氧化:一种途径涉及CO的直接转化L(线性键合的CO)转化为CO 2,而第二步通过AAL(乙醛)转化为CO L,然后再转化为CO 2进行,第三步通过吸附的乙酸盐形成AA(乙酸),可能是由AAL生产。相对于Pt / C,这种机制在合金的负电位更高时促进了EOR,还提供了更高的电流密度。因此,在Pt结构中包含Sn增强了在酸性介质中对EOR的催化活性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号