Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2018-08-27 , DOI: 10.1016/j.bmcl.2018.08.031 Akihiro Suemasa , Mizuki Watanabe , Takaaki Kobayashi , Hiroe Suzuki , Hayato Fukuda , Masabumi Minami , Satoshi Shuto
|
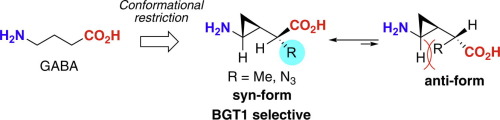
We previously designed and synthesized a series of cyclopropane-based conformationally restricted analogues of γ-aminobutyric acid (GABA). The study demonstrated that the critical conformation of the analogues that selectively active to betaine/GABA transporter 1 (BGT1) subtype is the trans-syn-form, in which the amino and carboxyl groups are in trans-configuration and the cyclopropane ring and the carboxyl group are in syn-arrangement. In this study, we designed and synthesized cyclopropane-based GABA analogues, which were conformationally restricted in the trans-syn-form by cyclopropylic strain based on the stereochemistry of the carbon adjacent to cyclopropane. Their conformation was confirmed as the syn-form by calculations and NMR studies, and their pharmacological evaluation clarified that compounds 11a and 11d had the BGT1 selectivity, although their inhibitory effects were insufficient.
中文翻译:

设计和合成基于环丙烷构象限制的GABA类似物作为甜菜碱/ GABA转运蛋白1的选择性抑制剂
我们以前设计和合成了一系列基于环丙烷的构象受限的γ-氨基丁酸(GABA)。研究表明,对甜菜碱/ GABA转运蛋白1(BGT1)亚型具有选择性活性的类似物的关键构象是反式合成形式,其中氨基和羧基处于反式构型,环丙烷环和羧基小组是同步安排的。在这项研究中,我们设计和合成了基于环丙烷的GABA类似物,该结构基于与环丙烷相邻的碳的立体化学,在构型上受到环丙基菌株的反式-顺式限制。他们的构象被确认为通过计算和NMR研究证实其具有顺式-形式,并且它们的药理学评价表明,化合物11a和11d具有BGT1选择性,尽管它们的抑制作用不足。











































 京公网安备 11010802027423号
京公网安备 11010802027423号