当前位置:
X-MOL 学术
›
Chem. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Search and Subvert: Minimalist Bacterial Phosphatidylinositol-Specific Phospholipase C Enzymes
Chemical Reviews ( IF 51.4 ) Pub Date : 2018-08-27 00:00:00 , DOI: 10.1021/acs.chemrev.8b00208 Mary F. Roberts 1 , Hanif M. Khan , Rebecca Goldstein 1 , Nathalie Reuter , Anne Gershenson 2
Chemical Reviews ( IF 51.4 ) Pub Date : 2018-08-27 00:00:00 , DOI: 10.1021/acs.chemrev.8b00208 Mary F. Roberts 1 , Hanif M. Khan , Rebecca Goldstein 1 , Nathalie Reuter , Anne Gershenson 2
Affiliation
|
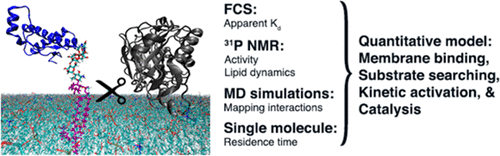
Phosphatidylinositol-specific phospholipase C (PI-PLC) enzymes from Gram-positive bacteria are secreted virulence factors that aid in downregulating host immunity. These PI-PLCs are minimalist peripheral membrane enzymes with a distorted (βα)8 TIM barrel fold offering a conserved and stable scaffold for the conserved catalytic amino acids while membrane recognition is achieved mostly through variable loops. Decades of experimental and computational research on these enzymes have revealed the subtle interplay between molecular mechanisms of catalysis and membrane binding, leading to a semiquantitative model for how they find, bind, and cleave their respective substrates on host cell membranes. Variations in sequence and structure of their membrane binding sites may correlate with how enzymes from different Gram-positive bacteria search for their particular targets on the membrane. Detailed molecular characterization of protein–lipid interactions have been aided by cutting-edge methods ranging from 31P field-cycling NMR relaxometry to monitor protein-induced changes in phospholipid dynamics to molecular dynamics simulations to elucidate the roles of electrostatic and cation−π interactions in lipid binding to single molecule fluorescence measurements of dynamic interactions between PI-PLCs and vesicles. This toolkit is readily applicable to other peripheral membrane proteins including orthologues in Gram-negative bacteria and more recently discovered eukaryotic minimalist PI-PLCs.
中文翻译:

搜索和颠覆:极简主义的细菌磷脂酰肌醇特异性磷脂酶C酶
来自革兰氏阳性细菌的磷脂酰肌醇特异性磷脂酶C(PI-PLC)酶是分泌的毒力因子,有助于下调宿主免疫力。这些PI-PLC是极简的外周膜酶,具有扭曲的(βα)8TIM桶折叠为保守的催化氨基酸提供了一个稳定而稳定的支架,而膜识别主要是通过可变环实现的。对这些酶的数十年实验和计算研究揭示了催化的分子机制与膜结合之间的微妙相互作用,从而导致了它们在宿主细胞膜上如何发现,结合和裂解其各自底物的半定量模型。它们的膜结合位点的序列和结构的变化可能与来自不同革兰氏阳性细菌的酶如何在膜上搜索其特定靶标有关。蛋白质-脂质相互作用的详细分子表征已通过最先进的方法得到了帮助,范围从31P场循环NMR弛豫法可监测蛋白质诱导的磷脂动力学变化,以进行分子动力学模拟,以阐明静电和阳离子-π相互作用在脂质结合中的作用,以结合PI-PLC和囊泡之间的相互作用的单分子荧光测量。该工具包可轻松应用于其他外周膜蛋白,包括革兰氏阴性细菌中的直向同源物和最近发现的真核极简主义PI-PLC。
更新日期:2018-08-27
中文翻译:

搜索和颠覆:极简主义的细菌磷脂酰肌醇特异性磷脂酶C酶
来自革兰氏阳性细菌的磷脂酰肌醇特异性磷脂酶C(PI-PLC)酶是分泌的毒力因子,有助于下调宿主免疫力。这些PI-PLC是极简的外周膜酶,具有扭曲的(βα)8TIM桶折叠为保守的催化氨基酸提供了一个稳定而稳定的支架,而膜识别主要是通过可变环实现的。对这些酶的数十年实验和计算研究揭示了催化的分子机制与膜结合之间的微妙相互作用,从而导致了它们在宿主细胞膜上如何发现,结合和裂解其各自底物的半定量模型。它们的膜结合位点的序列和结构的变化可能与来自不同革兰氏阳性细菌的酶如何在膜上搜索其特定靶标有关。蛋白质-脂质相互作用的详细分子表征已通过最先进的方法得到了帮助,范围从31P场循环NMR弛豫法可监测蛋白质诱导的磷脂动力学变化,以进行分子动力学模拟,以阐明静电和阳离子-π相互作用在脂质结合中的作用,以结合PI-PLC和囊泡之间的相互作用的单分子荧光测量。该工具包可轻松应用于其他外周膜蛋白,包括革兰氏阴性细菌中的直向同源物和最近发现的真核极简主义PI-PLC。











































 京公网安备 11010802027423号
京公网安备 11010802027423号