当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unravelling the origins of ice nucleation on organic crystals†
Chemical Science ( IF 7.6 ) Pub Date : 2018-08-27 00:00:00 , DOI: 10.1039/c8sc02753f Gabriele C Sosso 1 , Thomas F Whale 2 , Mark A Holden 2, 3 , Philipp Pedevilla 4 , Benjamin J Murray 2 , Angelos Michaelides 4
Chemical Science ( IF 7.6 ) Pub Date : 2018-08-27 00:00:00 , DOI: 10.1039/c8sc02753f Gabriele C Sosso 1 , Thomas F Whale 2 , Mark A Holden 2, 3 , Philipp Pedevilla 4 , Benjamin J Murray 2 , Angelos Michaelides 4
Affiliation
|
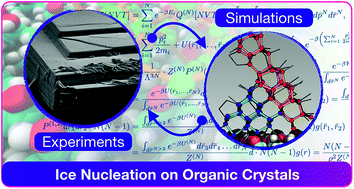
Organic molecules such as steroids or amino acids form crystals that can facilitate the formation of ice – arguably the most important phase transition on earth. However, the origin of the ice nucleating ability of organic crystals is still largely unknown. Here, we combine experiments and simulations to unravel the microscopic details of ice formation on cholesterol, a prototypical organic crystal widely used in cryopreservation. We find that cholesterol – which is also a substantial component of cell membranes – is an ice nucleating agent more potent than many inorganic substrates, including the mineral feldspar (one of the most active ice nucleating materials in the atmosphere). Scanning electron microscopy measurements reveal a variety of morphological features on the surfaces of cholesterol crystals: this suggests that the topography of the surface is key to the broad range of ice nucleating activity observed (from −4 to −20 °C). In addition, we show via molecular simulations that cholesterol crystals aid the formation of ice nuclei in a unconventional fashion. Rather than providing a template for a flat ice-like contact layer (as found in the case of many inorganic substrates), the flexibility of the cholesterol surface and its low density of hydrophilic functional groups leads to the formation of molecular cages involving both water molecules and terminal hydroxyl groups of the cholesterol surface. These cages are made of 6- and, surprisingly, 5-membered hydrogen bonded rings of water and hydroxyl groups that favour the nucleation of hexagonal as well as cubic ice (a rare occurrence). We argue that the phenomenal ice nucleating activity of steroids such as cholesterol (and potentially of many other organic crystals) is due to (i) the ability of flexible hydrophilic surfaces to form unconventional ice-templating structures and (ii) the different nucleation sites offered by the diverse topography of the crystalline surfaces. These findings clarify how exactly organic crystals promote the formation of ice, thus paving the way toward deeper understanding of ice formation in soft and biological matter – with obvious reverberations on atmospheric science and cryobiology.
中文翻译:

揭示有机晶体上冰成核的起源†
类固醇或氨基酸等有机分子形成的晶体可以促进冰的形成——可以说是地球上最重要的相变。然而,有机晶体的冰成核能力的起源仍然很大程度上未知。在这里,我们结合实验和模拟来揭示胆固醇冰形成的微观细节,胆固醇是一种广泛用于冷冻保存的典型有机晶体。我们发现胆固醇(也是细胞膜的重要组成部分)是一种比许多无机基质更有效的冰成核剂,包括矿物长石(大气中最活跃的冰成核材料之一)。扫描电子显微镜测量揭示了胆固醇晶体表面的各种形态特征:这表明表面形貌是观察到的广泛冰成核活动(-4 至 -20 °C)的关键。此外,我们通过分子模拟表明,胆固醇晶体以非常规的方式帮助冰核的形成。胆固醇表面的灵活性及其低密度的亲水官能团导致形成涉及水分子的分子笼,而不是为平坦的冰状接触层提供模板(如许多无机基材的情况)和胆固醇表面的末端羟基。这些笼子由水和羟基的 6 元氢键环和令人惊讶的 5 元氢键环组成,有利于六方冰和立方冰的成核(罕见)。我们认为,胆固醇等类固醇(以及可能的许多其他有机晶体)的惊人冰成核活性是由于(i)柔性亲水表面形成非常规冰模板结构的能力和(ii)提供的不同成核位点通过晶体表面的不同形貌。这些发现阐明了有机晶体究竟如何促进冰的形成,从而为更深入地了解软物质和生物物质中的冰形成铺平了道路,这对大气科学和低温生物学产生了明显的影响。
更新日期:2018-08-27
中文翻译:

揭示有机晶体上冰成核的起源†
类固醇或氨基酸等有机分子形成的晶体可以促进冰的形成——可以说是地球上最重要的相变。然而,有机晶体的冰成核能力的起源仍然很大程度上未知。在这里,我们结合实验和模拟来揭示胆固醇冰形成的微观细节,胆固醇是一种广泛用于冷冻保存的典型有机晶体。我们发现胆固醇(也是细胞膜的重要组成部分)是一种比许多无机基质更有效的冰成核剂,包括矿物长石(大气中最活跃的冰成核材料之一)。扫描电子显微镜测量揭示了胆固醇晶体表面的各种形态特征:这表明表面形貌是观察到的广泛冰成核活动(-4 至 -20 °C)的关键。此外,我们通过分子模拟表明,胆固醇晶体以非常规的方式帮助冰核的形成。胆固醇表面的灵活性及其低密度的亲水官能团导致形成涉及水分子的分子笼,而不是为平坦的冰状接触层提供模板(如许多无机基材的情况)和胆固醇表面的末端羟基。这些笼子由水和羟基的 6 元氢键环和令人惊讶的 5 元氢键环组成,有利于六方冰和立方冰的成核(罕见)。我们认为,胆固醇等类固醇(以及可能的许多其他有机晶体)的惊人冰成核活性是由于(i)柔性亲水表面形成非常规冰模板结构的能力和(ii)提供的不同成核位点通过晶体表面的不同形貌。这些发现阐明了有机晶体究竟如何促进冰的形成,从而为更深入地了解软物质和生物物质中的冰形成铺平了道路,这对大气科学和低温生物学产生了明显的影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号