当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Intramolecular [3 + 2] Cycloaddition Reactions of Unsaturated Nitrile Oxides. A Study from the Perspective of Bond Evolution Theory (BET)
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-08-24 00:00:00 , DOI: 10.1021/acs.jpca.8b06711 Abel Idrice Adjieufack 1, 2 , Vincent Liégeois 2 , Ibrahim Ndassa Mboumbouo 3 , Joseph Ketcha Mbadcam 1 , Benoît Champagne 2
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-08-24 00:00:00 , DOI: 10.1021/acs.jpca.8b06711 Abel Idrice Adjieufack 1, 2 , Vincent Liégeois 2 , Ibrahim Ndassa Mboumbouo 3 , Joseph Ketcha Mbadcam 1 , Benoît Champagne 2
Affiliation
|
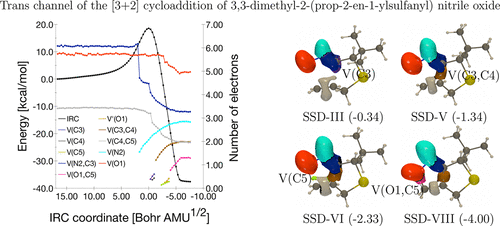
The reaction mechanism of the [3 + 2] intramolecular cycloaddition of 3,3-dimethyl-2-(prop-2-en-1-yloxy) and (prop-2-en-1-ylsulfanyl) nitrile oxides is analyzed using different DFT functionals with the 6-311++G(d,p) basis set. The activation and the reaction energies for the cis and trans pathways are evaluated at the DFT, MP2, and CCSD(T) levels of theory as well as their Gibbs free energy counterparts. It is shown that the trans regioisomers are both thermodynamic and kinetic compounds, in agreement with experimental outcomes. For a deeper understanding of the reaction mechanism, a BET analysis along the reaction channel (trans and cis) has been carried out. This analysis reveals that the lone pair on the nitrogen atom is formed first, then the C–C bond, and finally the O–C one. The global mechanism is similar for the two compounds and for the two pathways even if some small differences are observed, for instance, in the values of the reaction coordinates of appeareance of the different basins.
中文翻译:

不饱和腈氧化物的分子内[3 + 2]环加成反应。债券演化理论(BET)视角下的研究
3,3-二甲基-2-(丙-2-烯-1-基氧基)和(丙-2-烯-1-基硫烷基)腈氧化物的[3 + 2]分子内环加成反应机理用不同的方法分析具有6-311 ++ G(d,p)基础集的DFT功能。在理论的DFT,MP2和CCSD(T)以及它们的Gibbs自由能对应物的水平上评估顺式和反式途径的活化和反应能。结果表明,反式区域异构体是热力学和动力学化合物,与实验结果一致。对于该反应机理的更深入的了解,沿着所述反应通道的BET分析(反式和顺)已执行。该分析表明,氮原子上的孤对首先形成,然后是C-C键,最后是O-C键。即使观察到一些小的差异(例如,在不同盆地出现的反应坐标值方面),这两种化合物和这两种途径的总体机理也相似。
更新日期:2018-08-24
中文翻译:

不饱和腈氧化物的分子内[3 + 2]环加成反应。债券演化理论(BET)视角下的研究
3,3-二甲基-2-(丙-2-烯-1-基氧基)和(丙-2-烯-1-基硫烷基)腈氧化物的[3 + 2]分子内环加成反应机理用不同的方法分析具有6-311 ++ G(d,p)基础集的DFT功能。在理论的DFT,MP2和CCSD(T)以及它们的Gibbs自由能对应物的水平上评估顺式和反式途径的活化和反应能。结果表明,反式区域异构体是热力学和动力学化合物,与实验结果一致。对于该反应机理的更深入的了解,沿着所述反应通道的BET分析(反式和顺)已执行。该分析表明,氮原子上的孤对首先形成,然后是C-C键,最后是O-C键。即使观察到一些小的差异(例如,在不同盆地出现的反应坐标值方面),这两种化合物和这两种途径的总体机理也相似。









































 京公网安备 11010802027423号
京公网安备 11010802027423号