当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Influence of Alkyne and Azide Substituents on the Choice of the Reaction Mechanism of the Cu+-Catalyzed Addition of Azides to Iodoalkynes
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-08-24 00:00:00 , DOI: 10.1021/acs.jpca.8b06894 Pedro J. Silva 1 , Carlos E. P. Bernardo 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-08-24 00:00:00 , DOI: 10.1021/acs.jpca.8b06894 Pedro J. Silva 1 , Carlos E. P. Bernardo 1
Affiliation
|
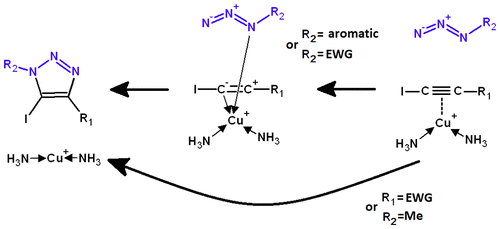
The cycloaddition of azides to iodoalkynes is strongly enhanced by some Cu+-complexes. We have studied computationally six reaction pathways for the cycloaddition of 24 combinations of azide and iodoalkyne to identify the dominant pathways and the influence of reactant structure on the evolution of the reaction. Two pathways were found to be operating for distinct sets of reactants. In the first pathway, initial complexation of iodoalkyne by Cu+ is followed by the binding of the azide to the metal through its substituted nitrogen atom, followed by attack of the nonhalogenated alkyne carbon by the terminal nitrogen atom. This pathway is generally followed by aromatic or electron-deficient azides, unless the iodoalkyne bears an electron-withdrawing group. The second pathway is a single-step mechanism similar (apart from the alkyne bond weakening caused by complexation) to that observed in the absence of catalyst. Electron-deficient iodoalkynes and methyl azides strongly prefer this mechanism, regardless of the identity of the reaction partners. The catalytic gain obtained through the use of Cu+ depends only partially on its direct effect on the energy of the transition state (relative to that of the infinitely separated reactants) and may be lost if the iodoalkyne itself strongly interacts with the catalyst through the formation of too strong a π-complex.
中文翻译:

炔和叠氮化物取代基对Cu +催化叠氮化物与碘代炔烃反应机理选择的影响
某些Cu + -配合物可大大增强叠氮化物与碘代炔烃的环加成反应。我们已经通过计算研究了用于叠氮化物和碘代炔烃的24种组合的环加成反应的六个反应途径,以确定主要途径以及反应物结构对反应演化的影响。发现有两种途径对不同组的反应物起作用。在第一个途径中,Cu +对碘炔的初始络合接着是叠氮化物通过其取代的氮原子与金属结合,接着是非卤代炔烃碳被末端氮原子侵蚀。除非碘炔具有一个吸电子基团,否则通常在此途径之后是芳族或电子不足的叠氮化物。第二种途径是类似于在不存在催化剂的情况下观察到的单步机理(除了由于络合引起的炔键减弱)。缺乏电子的碘代炔烃和叠氮化物强烈喜欢这种机理,而不管反应伙伴的身份如何。通过使用Cu +获得的催化增益 它仅部分取决于其对过渡态能量的直接影响(相对于无限分离的反应物的能量),如果碘炔本身通过形成太强的π络合物与催化剂强烈相互作用,则可能会丢失。
更新日期:2018-08-24
中文翻译:

炔和叠氮化物取代基对Cu +催化叠氮化物与碘代炔烃反应机理选择的影响
某些Cu + -配合物可大大增强叠氮化物与碘代炔烃的环加成反应。我们已经通过计算研究了用于叠氮化物和碘代炔烃的24种组合的环加成反应的六个反应途径,以确定主要途径以及反应物结构对反应演化的影响。发现有两种途径对不同组的反应物起作用。在第一个途径中,Cu +对碘炔的初始络合接着是叠氮化物通过其取代的氮原子与金属结合,接着是非卤代炔烃碳被末端氮原子侵蚀。除非碘炔具有一个吸电子基团,否则通常在此途径之后是芳族或电子不足的叠氮化物。第二种途径是类似于在不存在催化剂的情况下观察到的单步机理(除了由于络合引起的炔键减弱)。缺乏电子的碘代炔烃和叠氮化物强烈喜欢这种机理,而不管反应伙伴的身份如何。通过使用Cu +获得的催化增益 它仅部分取决于其对过渡态能量的直接影响(相对于无限分离的反应物的能量),如果碘炔本身通过形成太强的π络合物与催化剂强烈相互作用,则可能会丢失。











































 京公网安备 11010802027423号
京公网安备 11010802027423号