Microporous and Mesoporous Materials ( IF 4.8 ) Pub Date : 2018-08-26 , DOI: 10.1016/j.micromeso.2018.08.026 Fangfang Su , Qiaojuan Jia , Zhenzhen Li , Minghua Wang , Linghao He , Donglai Peng , Yingpan Song , Zhihong Zhang , Shaoming Fang
|
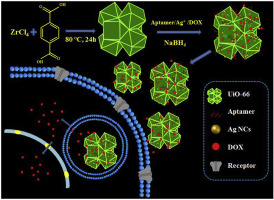
In this study, we reported a targeted antitumor drug delivery system (DDS) based on the nanocomposite of zirconium metal-organic framework (Zr-MOF, UiO-66) embedded with bioactive silver nanoclusters (Ag NCs) by using AS1411 aptamer (Apt) as the template (denoted by [email protected]@Apt). Targeted antitumor drug delivery system [email protected]@[email protected] was also obtained by one-pot encapsulation of antitumor drug doxorubicin (DOX) and formation of Ag NCs, which showed higher DOX loading efficiency and sustained controlled release than the [email protected]@Apt/DOX formed by two separate processes. A proof-of-principle targeting specificity study conducted by confocal laser scanning microscopy reveals that AS1411 aptamer-modified [email protected]@Apt can be effectively taken up and internalized by target cancer cells with high selectivity. In vitro cellular uptake and drug delivery study were further compared for both cancer MCF-7 and normal L929 cells to validate the enhanced tumor-targeted delivery of DOX. The results show that [email protected]@[email protected] can be internalized by AS1411-mediated endocytosis, and the released DOX can be effectively delivered to the nucleus, which can serve as in vivo targeted drug delivery system. Cell viability assay illustrates that the synthesized [email protected]@Apt nanocomposite possesses low cytotoxicity to MCF-7 cell in a wide concentration range of 5–50 μg mL−1, and the drug formulations exhibit good capability for targeted DOX delivery and intracellular controlled release, leading to a robust and enhanced antitumor effect in vitro. These results prove that the proposed AS1411-functionalized [email protected]@[email protected] can be a promising targeted drug delivery platform for cancer therapy.
中文翻译:

嵌入锆金属-有机框架中的适体模板银纳米簇可用于靶向抗肿瘤药物递送
在这项研究中,我们报道了使用AS1411适体(Apt)嵌入生物活性银纳米团簇(Ag NCs)的锆金属-有机骨架(Zr-MOF,UiO-66)纳米复合材料为基础的靶向抗肿瘤药物递送系统(DDS)。作为模板(由[电子邮件保护] @Apt表示)。还通过一锅封装抗肿瘤药物阿霉素(DOX)和形成Ag NCs获得了靶向抗肿瘤药物递送系统[电子邮件保护] @ [电子邮件保护],与[电子邮件保护]相比,它具有更高的DOX加载效率和持续控释。 ] @ Apt / DOX由两个独立的过程组成。通过共聚焦激光扫描显微镜进行的原理靶向特异性研究表明,AS1411适体修饰的[电子邮件保护] @Apt可以被高选择性的靶癌细胞有效吸收和内化。在进一步比较了癌症MCF-7细胞和正常L929细胞的体外细胞吸收和药物传递研究,以验证DOX增强了靶向肿瘤的传递。结果表明[电子邮件保护] @ [电子邮件保护]可以被AS1411-介导的胞吞作用内在化,释放的DOX可以有效地传递到细胞核,可以作为体内靶向药物传递系统。细胞活力测定表明,合成的[电子邮件保护] @Apt纳米复合材料在5–50μgmL -1的宽浓度范围内对MCF-7细胞具有低细胞毒性,并且这些药物制剂表现出良好的靶向DOX传递和细胞内控释的能力,从而在体外产生了强大且增强的抗肿瘤作用。这些结果证明,拟议的AS1411功能化[受电子邮件保护] @ [受电子邮件保护]可以成为有前途的针对癌症治疗的靶向药物递送平台。











































 京公网安备 11010802027423号
京公网安备 11010802027423号