当前位置:
X-MOL 学术
›
Catal. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oxygen-promoted Suzuki–Miyaura reaction of aryl fluorosulfates and potassium aryltrifluoroborates: Mild and efficient access to biaryls and terphenyls
Catalysis Communications ( IF 3.4 ) Pub Date : 2018-08-24 , DOI: 10.1016/j.catcom.2018.08.023 Xinmin Li , Yunhai Ma , Qinghong Hu , Bo Jiang , Qing Wu , Zeli Yuan
中文翻译:

氧促进芳基氟硫酸盐和芳基三氟硼酸钾的Suzuki-Miyaura反应:轻而有效地获得联芳基和三联苯
更新日期:2018-08-24
Catalysis Communications ( IF 3.4 ) Pub Date : 2018-08-24 , DOI: 10.1016/j.catcom.2018.08.023 Xinmin Li , Yunhai Ma , Qinghong Hu , Bo Jiang , Qing Wu , Zeli Yuan
|
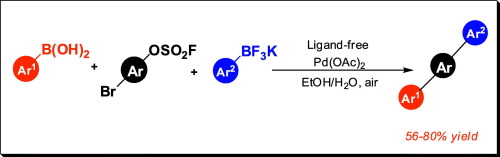
A mild and efficient protocol has been developed for the Suzuki–Miyaura reaction of aryl fluorosulfates and potassium aryltrifluoroborates at room temperature. A series of biaryls can be prepared with excellent yields in this system and an aerobic atmosphere demonstrates a positive effect on the reactivity of the cross-coupling reactions. Additionally, this method can be extended to one-pot double Suzuki–Miyaura reactions, allowing the synthesis of various unsymmetrical terphenyls in moderate to good yields.
中文翻译:

氧促进芳基氟硫酸盐和芳基三氟硼酸钾的Suzuki-Miyaura反应:轻而有效地获得联芳基和三联苯
对于室温下芳基氟硫酸盐和芳基三氟硼酸钾的Suzuki-Miyaura反应,已开发出温和有效的方案。在该体系中,可以制备出一系列具有优异收率的联芳基,好氧气氛对交叉偶联反应的反应性产生了积极的影响。此外,该方法可以扩展到一锅双Suzuki-Miyaura反应,从而可以中等至良好的产率合成各种不对称的三联苯。











































 京公网安备 11010802027423号
京公网安备 11010802027423号