当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Impact of Drug Physicochemical Properties on Lipolysis-Triggered Drug Supersaturation and Precipitation from Lipid-Based Formulations.
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2018-09-07 , DOI: 10.1021/acs.molpharmaceut.8b00699 Linda C Alskär 1 , Janneke Keemink 1 , Jenny Johannesson 1 , Christopher J H Porter 2 , Christel A S Bergström 1, 2
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2018-09-07 , DOI: 10.1021/acs.molpharmaceut.8b00699 Linda C Alskär 1 , Janneke Keemink 1 , Jenny Johannesson 1 , Christopher J H Porter 2 , Christel A S Bergström 1, 2
Affiliation
|
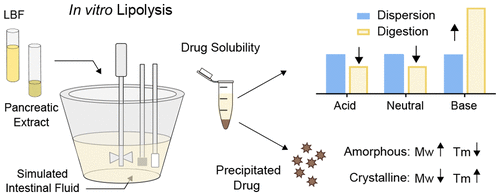
In this study we investigated lipolysis-triggered supersaturation and precipitation of a set of model compounds formulated in lipid-based formulations (LBFs). The purpose was to explore the relationship between precipitated solid form and inherent physicochemical properties of the drug. Eight drugs were studied after formulation in three LBFs, representing lipid-rich (extensively digestible) to surfactant-rich (less digestible) formulations. In vitro lipolysis of drug-loaded LBFs were conducted, and the amount of dissolved and precipitated drug was quantified. Solid form of the precipitated drug was characterized with polarized light microscopy (PLM) and Raman spectroscopy. A significant solubility increase for the weak bases in the presence of digestion products was observed, in contrast to the neutral and acidic compounds for which the solubility decreased. The fold-increase in solubility was linked to the degree of ionization of the weak bases and thus their attraction to free fatty acids. A high level of supersaturation was needed to cause precipitation. For the weak bases, the dose number indicated that precipitation would not occur during lipolysis; hence, these compounds were not included in further studies. The solid state analysis proved that danazol and griseofulvin precipitated in a crystalline form, while niclosamide precipitated as a hydrate. Felodipine and indomethacin crystals were visible in the PLM, whereas the Raman spectra showed presence of amorphous drug, indicating amorphous precipitation that quickly crystallized. The solid state analysis was combined with literature data to allow analysis of the relationship between solid form and the physicochemical properties of the drug. It was found that low molecular weight and high melting temperature increases the probability of crystalline precipitation, whereas precipitation in an amorphous form was favored by high molecular weight, low melting temperature, and positive charge.
中文翻译:

药物理化性质对脂基引发的脂解引发的药物过饱和和沉淀的影响。
在这项研究中,我们研究了脂解引发的过饱和度和在基于脂质的制剂(LBF)中配制的一组模型化合物的沉淀。目的是探索沉淀的固体形式与药物固有的物理化学性质之间的关系。在三个LBFs中配制后研究了八种药物,分别代表了富含脂质(可广泛消化)至富含表面活性剂(难以消化)的制剂。进行了载有药物的LBF的体外脂解,并对溶解和沉淀的药物量进行了定量。沉淀药物的固体形式通过偏振光显微镜(PLM)和拉曼光谱法表征。在消化产物存在下,弱碱的溶解度显着增加,与溶解度降低的中性和酸性化合物相反。溶解度的增加倍数与弱碱的离子化程度有关,因此与弱脂肪酸的吸引力有关。需要高水平的过饱和来引起沉淀。对于弱碱,剂量数表明在脂解过程中不会发生沉淀。因此,这些化合物未包括在进一步的研究中。固态分析证明,达那唑和灰黄霉素以结晶形式沉淀,而烟酰胺则以水合物沉淀。在PLM中可见非洛地平和消炎痛晶体,而拉曼光谱显示存在无定形药物,表明无定形沉淀迅速结晶。固态分析与文献数据相结合,可以分析固体形式与药物理化性质之间的关系。发现低分子量和高熔融温度增加了结晶沉淀的可能性,而高分子量,低熔融温度和正电荷有利于以无定形形式的沉淀。
更新日期:2018-08-24
中文翻译:

药物理化性质对脂基引发的脂解引发的药物过饱和和沉淀的影响。
在这项研究中,我们研究了脂解引发的过饱和度和在基于脂质的制剂(LBF)中配制的一组模型化合物的沉淀。目的是探索沉淀的固体形式与药物固有的物理化学性质之间的关系。在三个LBFs中配制后研究了八种药物,分别代表了富含脂质(可广泛消化)至富含表面活性剂(难以消化)的制剂。进行了载有药物的LBF的体外脂解,并对溶解和沉淀的药物量进行了定量。沉淀药物的固体形式通过偏振光显微镜(PLM)和拉曼光谱法表征。在消化产物存在下,弱碱的溶解度显着增加,与溶解度降低的中性和酸性化合物相反。溶解度的增加倍数与弱碱的离子化程度有关,因此与弱脂肪酸的吸引力有关。需要高水平的过饱和来引起沉淀。对于弱碱,剂量数表明在脂解过程中不会发生沉淀。因此,这些化合物未包括在进一步的研究中。固态分析证明,达那唑和灰黄霉素以结晶形式沉淀,而烟酰胺则以水合物沉淀。在PLM中可见非洛地平和消炎痛晶体,而拉曼光谱显示存在无定形药物,表明无定形沉淀迅速结晶。固态分析与文献数据相结合,可以分析固体形式与药物理化性质之间的关系。发现低分子量和高熔融温度增加了结晶沉淀的可能性,而高分子量,低熔融温度和正电荷有利于以无定形形式的沉淀。











































 京公网安备 11010802027423号
京公网安备 11010802027423号