当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The Tetrazole Analogue of the Auxin Indole-3-acetic Acid Binds Preferentially to TIR1 and Not AFB5
ACS Chemical Biology ( IF 4 ) Pub Date : 2018-08-23 00:00:00 , DOI: 10.1021/acschembio.8b00527 Mussa Quareshy , Justyna Prusinska , Martin Kieffer 1 , Kosuke Fukui 2 , Alonso J. Pardal , Silke Lehmann 3 , Patrick Schafer 3 , Charo I. del Genio , Stefan Kepinski 1 , Kenichiro Hayashi 2 , Andrew Marsh , Richard M. Napier
ACS Chemical Biology ( IF 4 ) Pub Date : 2018-08-23 00:00:00 , DOI: 10.1021/acschembio.8b00527 Mussa Quareshy , Justyna Prusinska , Martin Kieffer 1 , Kosuke Fukui 2 , Alonso J. Pardal , Silke Lehmann 3 , Patrick Schafer 3 , Charo I. del Genio , Stefan Kepinski 1 , Kenichiro Hayashi 2 , Andrew Marsh , Richard M. Napier
Affiliation
|
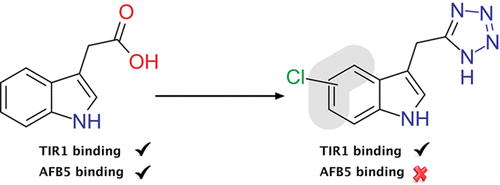
Indole-3-acetic acid (auxin) is considered one of the cardinal hormones in plant growth and development. It regulates a wide range of processes throughout the plant. Synthetic auxins exploit the auxin-signaling pathway and are valuable as herbicidal agrochemicals. Currently, despite a diversity of chemical scaffolds all synthetic auxins have a carboxylic acid as the active core group. By applying bio-isosteric replacement we discovered that indole-3-tetrazole was active by surface plasmon resonance spectrometry, showing that the tetrazole could initiate assembly of the Transport Inhibitor Resistant 1 (TIR1) auxin coreceptor complex. We then tested the tetrazole’s efficacy in a range of whole plant physiological assays and in protoplast reporter assays, which all confirmed auxin activity, albeit rather weak. We then tested indole-3-tetrazole against the AFB5 homologue of TIR1, finding that binding was selective against TIR1, absent with AFB5. The kinetics of binding to TIR1 are contrasted to those for the herbicide picloram, which shows the opposite receptor preference, as it binds to AFB5 with far greater affinity than to TIR1. The basis of the preference of indole-3-tetrazole for TIR1 was revealed to be a single residue substitution using molecular docking, and assays using tir1 and afb5 mutant lines confirmed selectivity in vivo. Given the potential that a TIR1-selective auxin might have for unmasking receptor-specific actions, we followed a rational design, lead optimization campaign, and a set of chlorinated indole-3-tetrazoles was synthesized. Improved affinity for TIR1 and the preference for binding to TIR1 was maintained for 4- and 6-chloroindole-3-tetrazoles, coupled with improved efficacy in vivo. This work expands the range of auxin chemistry for the design of receptor-selective synthetic auxins.
中文翻译:

生长素吲哚-3-乙酸的四唑类似物优先与TIR1结合而不与AFB5结合
吲哚-3-乙酸(植物生长素)被认为是植物生长发育的主要激素之一。它可以调节整个工厂内的各种过程。合成的植物生长素利用植物生长素的信号通路,作为除草剂农药非常有价值。当前,尽管化学支架多种多样,所有合成的植物生长素都具有羧酸作为活性核心基团。通过应用生物等排代换,我们发现吲哚-3-四唑通过表面等离振子共振光谱法具有活性,表明四唑可以启动抗运输转运蛋白1(TIR1)生长素共受体复合物的组装。然后,我们在一系列全植物生理学测定和原生质体记者测定中测试了四唑的功效,这些测定均证实了植物生长素的活性,尽管作用较弱。然后,我们针对TIR1的AFB5同源物测试了吲哚-3-四唑,发现结合对TIR1是选择性的,而AFB5则没有。与TIR1结合的动力学与除草剂吡氯吡喃的动力学相反,后者显示出相反的受体偏好,因为它以远大于TIR1的亲和力与AFB5结合。吲哚-3-四唑对TIR1的偏爱基础表明是使用分子对接的单个残基取代,以及使用TIR1的测定tir1和afb5突变株证实了体内的选择性。考虑到TIR1选择性生长素可能具有掩盖受体特异性作用的潜力,我们遵循合理的设计,进行了优化,并合成了一组氯化吲哚-3-四唑。对于4-和6-氯吲哚-3-四唑,维持了对TIR1的改善的亲和性和与TIR1结合的偏好,以及体内的改善的功效。这项工作扩大了用于设计受体选择性合成植物生长素的植物生长素化学范围。
更新日期:2018-08-23
中文翻译:

生长素吲哚-3-乙酸的四唑类似物优先与TIR1结合而不与AFB5结合
吲哚-3-乙酸(植物生长素)被认为是植物生长发育的主要激素之一。它可以调节整个工厂内的各种过程。合成的植物生长素利用植物生长素的信号通路,作为除草剂农药非常有价值。当前,尽管化学支架多种多样,所有合成的植物生长素都具有羧酸作为活性核心基团。通过应用生物等排代换,我们发现吲哚-3-四唑通过表面等离振子共振光谱法具有活性,表明四唑可以启动抗运输转运蛋白1(TIR1)生长素共受体复合物的组装。然后,我们在一系列全植物生理学测定和原生质体记者测定中测试了四唑的功效,这些测定均证实了植物生长素的活性,尽管作用较弱。然后,我们针对TIR1的AFB5同源物测试了吲哚-3-四唑,发现结合对TIR1是选择性的,而AFB5则没有。与TIR1结合的动力学与除草剂吡氯吡喃的动力学相反,后者显示出相反的受体偏好,因为它以远大于TIR1的亲和力与AFB5结合。吲哚-3-四唑对TIR1的偏爱基础表明是使用分子对接的单个残基取代,以及使用TIR1的测定tir1和afb5突变株证实了体内的选择性。考虑到TIR1选择性生长素可能具有掩盖受体特异性作用的潜力,我们遵循合理的设计,进行了优化,并合成了一组氯化吲哚-3-四唑。对于4-和6-氯吲哚-3-四唑,维持了对TIR1的改善的亲和性和与TIR1结合的偏好,以及体内的改善的功效。这项工作扩大了用于设计受体选择性合成植物生长素的植物生长素化学范围。



























 京公网安备 11010802027423号
京公网安备 11010802027423号