当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reprogramming a Deubiquitinase into a Transamidase
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2018-08-23 00:00:00 , DOI: 10.1021/acschembio.8b00759 Lin Hui Chang , Eric R. Strieter
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2018-08-23 00:00:00 , DOI: 10.1021/acschembio.8b00759 Lin Hui Chang , Eric R. Strieter
|
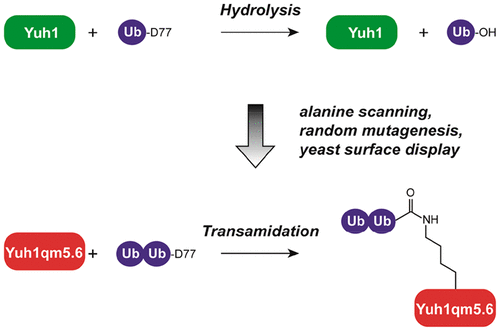
Access to well-defined ubiquitin conjugates has been key to elucidating the biochemical functions of proteins in the ubiquitin signaling network. Yet, we have a poor understanding of how deubiquitinases and ubiquitin-binding proteins respond to ubiquitin modifications when anchored to a protein other than ubiquitin or a ubiquitin-like protein. This is due to the difficulty of synthesizing ubiquitinated proteins comprised of native isopeptide bonds. Here we report on the evolution of a deubiquitinase capable of site-specifically modifying itself with defined ubiquitin chains. Following mutagenesis and yeast display screening, we identify a variant of the yeast ubiquitin C-terminal hydrolase Yuh1 that has a 28-fold improvement in the transamidation to hydrolysis ratio relative to the wild type enzyme. The switch in activity enables robust autoubiquitination of a lysine in the crossover loop to form an isopeptide bond. We demonstrate the utility of autoubiquitinating the evolved Yuh1 variant by investigating the consequences of ubiquitin chain anchoring on the activities of other deubiquitinases. Much to our surprise, we find that certain deubiquitinases are exquisitely sensitive to chain anchoring. These results highlight the importance of investigating the biochemical activities of deubiquitinases with both substrate-anchored and unanchored ubiquitin chains.
中文翻译:

将去泛素酶重编程为转酰胺酶
获得明确的泛素偶联物已成为阐明蛋白质在泛素信号网络中的生化功能的关键。然而,我们对除泛素化酶和泛素结合蛋白在锚定于除泛素或类似泛素的蛋白以外的蛋白时如何对泛素修饰的反应了解甚少。这是由于难以合成由天然异肽键组成的泛素化蛋白质。在这里,我们报道了一种去泛素化酶的进化过程,该酶能够通过定义的泛素链进行位点特异性修饰。在诱变和酵母展示筛选之后,我们确定了酵母泛素C末端水解酶Yuh1的变体,相对于野生型酶,其转酰胺基水解率提高了28倍。活性的改变使得赖氨酸在交叉环中强大的自泛素化形成异肽键。我们通过研究泛素链锚定对其他去泛素酶活性的影响,证明了自身泛素化进化的Yuh1变体的效用。令我们惊讶的是,我们发现某些去泛素酶对链锚定非常敏感。这些结果突出了研究底物锚定的和未锚定的遍在蛋白链的去泛素酶的生物化学活性的重要性。我们发现某些去泛素酶对链锚定非常敏感。这些结果突出了研究底物锚定的和未锚定的遍在蛋白链的去泛素酶的生物化学活性的重要性。我们发现某些去泛素酶对链锚定非常敏感。这些结果突出了研究底物锚定的和未锚定的遍在蛋白链的去泛素酶的生物化学活性的重要性。
更新日期:2018-08-23
中文翻译:

将去泛素酶重编程为转酰胺酶
获得明确的泛素偶联物已成为阐明蛋白质在泛素信号网络中的生化功能的关键。然而,我们对除泛素化酶和泛素结合蛋白在锚定于除泛素或类似泛素的蛋白以外的蛋白时如何对泛素修饰的反应了解甚少。这是由于难以合成由天然异肽键组成的泛素化蛋白质。在这里,我们报道了一种去泛素化酶的进化过程,该酶能够通过定义的泛素链进行位点特异性修饰。在诱变和酵母展示筛选之后,我们确定了酵母泛素C末端水解酶Yuh1的变体,相对于野生型酶,其转酰胺基水解率提高了28倍。活性的改变使得赖氨酸在交叉环中强大的自泛素化形成异肽键。我们通过研究泛素链锚定对其他去泛素酶活性的影响,证明了自身泛素化进化的Yuh1变体的效用。令我们惊讶的是,我们发现某些去泛素酶对链锚定非常敏感。这些结果突出了研究底物锚定的和未锚定的遍在蛋白链的去泛素酶的生物化学活性的重要性。我们发现某些去泛素酶对链锚定非常敏感。这些结果突出了研究底物锚定的和未锚定的遍在蛋白链的去泛素酶的生物化学活性的重要性。我们发现某些去泛素酶对链锚定非常敏感。这些结果突出了研究底物锚定的和未锚定的遍在蛋白链的去泛素酶的生物化学活性的重要性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号