当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetics of the Reaction of OH with Isoprene over a Wide Range of Temperature and Pressure Including Direct Observation of Equilibrium with the OH Adducts
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-08-23 00:00:00 , DOI: 10.1021/acs.jpca.8b04829 D. J. Medeiros 1 , M. A. Blitz 1, 2 , L. James 1 , T. H. Speak 1 , P. W. Seakins 1, 2
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-08-23 00:00:00 , DOI: 10.1021/acs.jpca.8b04829 D. J. Medeiros 1 , M. A. Blitz 1, 2 , L. James 1 , T. H. Speak 1 , P. W. Seakins 1, 2
Affiliation
|
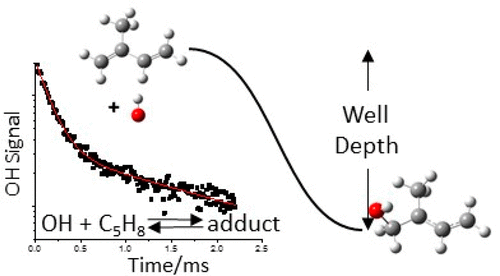
The reaction of the OH radical with isoprene, C5H8 (R1), has been studied over the temperature range 298–794 K and bath gas pressures of nitrogen from 50 to 1670 Torr using laser flash photolysis (LFP) to generate OH and laser-induced fluorescence (LIF) to observe OH removal. Measurements have been made using both a conventional LFP/LIF apparatus and a new high pressure system. The measured rate coefficient at 298 K (k1,298K = (9.90 ± 0.09) × 10–11 cm3 molecule–1 s–1) in the high pressure apparatus is in excellent agreement with the literature, confirming the accuracy of measurements made with this instrument. Above 700 K, the OH decays were no longer single exponentials due to regeneration of OH from adduct decomposition and the establishment of the OH + C5H8 ⇌ HOC5H8 equilibrium (R1a, R-1a). This equilibrium was analyzed by comparison to a master equation model of reaction R1 and determined the well depth for OH addition to carbon C1 and C4 to be equal to 153.5 ± 6.2 and 143.4 ± 6.2 kJ mol–1, respectively. These well depths are in excellent agreement with the present ab initio—CCSD(T)/CBS//M062X/6-311++G(3df,2p)—calculations (154.1 kJ mol–1 for the C1 adduct). Addition to the less stable C2 and C3 adducts is not important. The data above 700 K also indicated that a minor but significant direct abstraction channel, R1b, was also operating with k1b = (1.3 ± 0.3) × 10–11 exp(−3.61 kJ mol–1/RT) cm3 molecule–1 s–1. Additional support for the presence of this abstraction channel comes from our ab initio calculations and from room-temperature proton transfer mass spectrometry product analysis. The literature data on reaction R1 together with the present data were assessed using master equation analysis, using the MESMER package. This analysis produced a refined data set that generates our recommended k1a(T, [M]). An analytical representation of k1a(T, [M]) and k–1a(T, [M]) is provided via a Troe expression. The reported experimental data (the sum of addition and abstraction), k1∞ = (9.5 ± 0.2) × 10–11(T/298 K)−1.33±0.07 + (1.3 ± 0.3) × 10–11 exp(−3.61 kJ mol–1/RT) cm3 molecule–1 s–1, significantly extend the measured temperature range of R1.
中文翻译:

宽温度和压力范围内OH与异戊二烯反应的动力学,包括直接观察OH加合物的平衡
OH自由基与异戊二烯C 5 H 8(R1)的反应已在298–794 K的温度范围内和氮气的浴气压为50至1670 Torr的条件下进行了研究,方法是使用激光闪光光解(LFP)生成OH和激光诱导荧光(LIF)观察OH的去除。已经使用传统的LFP / LIF设备和新的高压系统进行了测量。在298 K(k 1,298K =(9.90±0.09)×10 –11 cm 3分子–1 s –1)在高压设备中与文献极为吻合,证实了使用该仪器进行测量的准确性。高于700 K,所述OH衰变不再单指数由于从加合物分解OH的再生和建立OH的+ C 5 H ^ 8 ⇌HOC 5 ħ 8平衡(R1A,R1A)。通过与反应R1的主方程模型进行比较,分析了该平衡,并确定了向碳C 1和C 4中添加OH的阱深度分别等于153.5±6.2和143.4±6.2 kJ mol –1。这些井深与目前的从头算是非常吻合的-CCSD(T)/ CBS // M062X / 6-311 ++ G(3df,2p)-计算值(对于C 1加合物为154.1 kJ mol –1)。除不稳定的C 2和C 3加合物外,并不重要。700 K以上的数据还表明,一个较小但重要的直接提取通道R1b也以k 1b =(1.3±0.3)×10 –11 exp(−3.61 kJ mol –1 / RT)cm 3分子–1运作s –1。从头开始,对存在此抽象通道的其他支持计算和从室温质子转移质谱产品分析。关于反应R1的文献数据以及本数据,通过使用MESMER软件包的主方程分析进行了评估。该分析产生了精确的数据集,该数据集生成了我们推荐的k 1a(T,[ M ])。通过Troe表达式提供了k 1a(T,[ M ])和k –1a(T,[ M ])的解析表示。所报告的实验数据,(加法和抽象的总和)ķ 1 ∞ =(9.5±0.2)×10 -11(T / 298 K)−1.33±0.07 +(1.3±0.3)×10 –11 exp(−3.61 kJ mol –1 / RT)cm 3分子–1 s –1,显着扩展了R1的测量温度范围。
更新日期:2018-08-23
中文翻译:

宽温度和压力范围内OH与异戊二烯反应的动力学,包括直接观察OH加合物的平衡
OH自由基与异戊二烯C 5 H 8(R1)的反应已在298–794 K的温度范围内和氮气的浴气压为50至1670 Torr的条件下进行了研究,方法是使用激光闪光光解(LFP)生成OH和激光诱导荧光(LIF)观察OH的去除。已经使用传统的LFP / LIF设备和新的高压系统进行了测量。在298 K(k 1,298K =(9.90±0.09)×10 –11 cm 3分子–1 s –1)在高压设备中与文献极为吻合,证实了使用该仪器进行测量的准确性。高于700 K,所述OH衰变不再单指数由于从加合物分解OH的再生和建立OH的+ C 5 H ^ 8 ⇌HOC 5 ħ 8平衡(R1A,R1A)。通过与反应R1的主方程模型进行比较,分析了该平衡,并确定了向碳C 1和C 4中添加OH的阱深度分别等于153.5±6.2和143.4±6.2 kJ mol –1。这些井深与目前的从头算是非常吻合的-CCSD(T)/ CBS // M062X / 6-311 ++ G(3df,2p)-计算值(对于C 1加合物为154.1 kJ mol –1)。除不稳定的C 2和C 3加合物外,并不重要。700 K以上的数据还表明,一个较小但重要的直接提取通道R1b也以k 1b =(1.3±0.3)×10 –11 exp(−3.61 kJ mol –1 / RT)cm 3分子–1运作s –1。从头开始,对存在此抽象通道的其他支持计算和从室温质子转移质谱产品分析。关于反应R1的文献数据以及本数据,通过使用MESMER软件包的主方程分析进行了评估。该分析产生了精确的数据集,该数据集生成了我们推荐的k 1a(T,[ M ])。通过Troe表达式提供了k 1a(T,[ M ])和k –1a(T,[ M ])的解析表示。所报告的实验数据,(加法和抽象的总和)ķ 1 ∞ =(9.5±0.2)×10 -11(T / 298 K)−1.33±0.07 +(1.3±0.3)×10 –11 exp(−3.61 kJ mol –1 / RT)cm 3分子–1 s –1,显着扩展了R1的测量温度范围。











































 京公网安备 11010802027423号
京公网安备 11010802027423号