Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2018-08-22 , DOI: 10.1016/j.bmcl.2018.08.021 Min Huang , Aihua Li , Feng Zhao , Xiaorui Xie , Kun Li , Yongkui Jing , Dan Liu , Linxiang Zhao
|
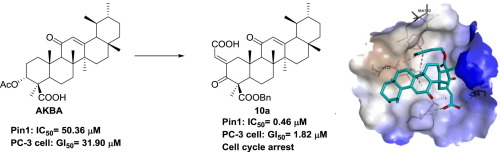
Pin1 (Protein interaction with never in mitosis A1) is a validated molecular target for anticancer drug discovery. Herein, we reported the design, synthesis, and structure-activity relationship study of novel ring A modified AKBA (3-acetyl-11-keto-boswellic acid) derivatives as Pin1 inhibitors. Most compounds showed superior Pin1 inhibitory activities to AKBA. One of the most promising compounds, 10a, potently inhibited Pin1 with IC50 value of 0.46 μM, while it displayed excellent anti-proliferative effect against prostate cancer cells PC-3 with GI50 value of 1.82 μM. Structure-activity relationship indicated that reasonable structural modifications in ring A had significant impact on improving activity. Further mechanism research revealed that 10a decreased the level of Cyclin D1 and caused cell cycle arrest at G0/G1 phase in PC-3 cancer cells. Thus, compound 10a may serve as potential anti-prostate cancer agent for further investigation through Pin1 inhibition.
中文翻译:

具有显着抗前列腺癌活性的Pin1抑制剂的环A修饰的11-酮-乳香酸衍生物的设计,合成和生物学评估
Pin1(蛋白质在有丝分裂A1中从未相互作用)是经过验证的抗癌药物分子靶标。在本文中,我们报道了作为Pin1抑制剂的新型A环修饰的AKBA(3-乙酰基-11-酮-乳香酸)衍生物的设计,合成和构效关系研究。大多数化合物显示出优于AKBA的Pin1抑制活性。最有前途的化合物之一10a可以有效地抑制Pin1,其IC 50值为0.46μM,而它对GI 50值为1.82μM的前列腺癌细胞PC-3表现出优异的抗增殖作用。结构-活性关系表明,环A中合理的结构修饰对改善活性具有重要影响。进一步的机理研究表明10a降低了PC-3癌细胞中Cyclin D1的水平并导致细胞周期停滞在G0 / G1期。因此,化合物10a可以作为潜在的抗前列腺癌药物,通过抑制Pin1进行进一步研究。











































 京公网安备 11010802027423号
京公网安备 11010802027423号