当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Transition-Metal (Cu, Pd, Ni)-Catalyzed Difluoroalkylation via Cross-Coupling with Difluoroalkyl Halides
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2018-08-22 00:00:00 , DOI: 10.1021/acs.accounts.8b00230 Zhang Feng 1 , Yu-Lan Xiao 1 , Xingang Zhang 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2018-08-22 00:00:00 , DOI: 10.1021/acs.accounts.8b00230 Zhang Feng 1 , Yu-Lan Xiao 1 , Xingang Zhang 1
Affiliation
|
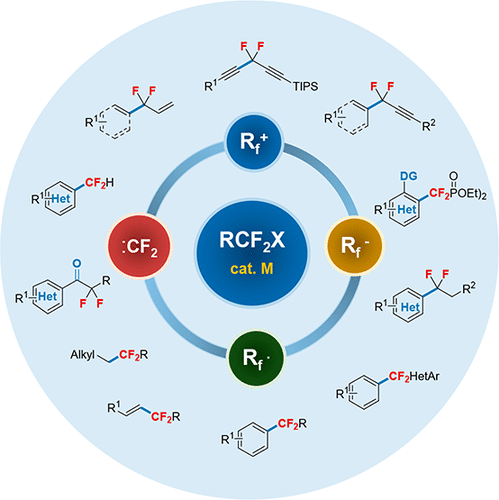
Difluoroalkylated compounds play a remarkably important role in life and materials sciences because of the unique characteristics of the difluoromethylene (CF2) group. In particular, precise introduction of a CF2 group at the benzylic position can dramatically improve the biological properties of the corresponding molecules. As a consequence, difluoroalkylation of aromatic compounds has become a powerful strategy in modulating the bioactivities of organic molecules. However, efficient strategies to selectively synthesize difluoroalkylated arenes had been very limited before 2012. Traditional synthetic methods in this regard suffer from either harsh reaction conditions or narrow substrate scope, significantly restricting their widespread applications, particularly for late-stage difluoroalkylation of bioactive molecules. To overcome these limitations, a straightforward route to access these valuable difluoroalkylated skeletons is the direct introduction of the difluoroalkylated group (CF2R) onto aromatic rings through transition-metal-catalyzed cross-coupling. However, because of the instability of some difluoroalkylated metal species, which are prone to protonation, dimerization, and/or generation of other unknown byproducts, it is difficult to selectively control the catalytic cycle to suppress these side reactions. In this context, we proposed the use of low-cost and widely available difluoroalkyl halides as fluoroalkyl sources for transition-metal-catalyzed difluoroalkylation reactions via cross-coupling.
中文翻译:

过渡金属(铜,钯,镍)与二氟烷基卤化物交叉偶联催化的二氟烷基化
由于二氟亚甲基(CF 2)基团的独特特性,二氟烷基化的化合物在生命和材料科学中起着非常重要的作用。特别是CF 2的精确引入苄基位置的基团可以显着改善相应分子的生物学特性。结果,芳族化合物的二氟烷基化已成为调节有机分子生物活性的有效策略。但是,2012年之前,选择性合成二氟烷基化芳烃的有效策略非常有限。传统的合成方法在苛刻的反应条件或狭窄的底物范围内都受到困扰,这极大地限制了它们的广泛应用,特别是对于生物活性分子的后期二氟烷基化。为了克服这些局限性,直接访问这些有价值的二氟烷基化骨架的方法是直接引入二氟烷基化基团(CF 2R)通过过渡金属催化的交叉偶联作用到芳环上。但是,由于某些易于被质子化,二聚化和/或生成其他未知副产物的二氟烷基化金属种类的不稳定性,难以选择性地控制催化循环以抑制这些副反应。在这种情况下,我们提出使用低成本和广泛使用的二氟烷基卤化物作为氟烷基源,用于通过交叉偶联进行过渡金属催化的二氟烷基化反应。
更新日期:2018-08-22
中文翻译:

过渡金属(铜,钯,镍)与二氟烷基卤化物交叉偶联催化的二氟烷基化
由于二氟亚甲基(CF 2)基团的独特特性,二氟烷基化的化合物在生命和材料科学中起着非常重要的作用。特别是CF 2的精确引入苄基位置的基团可以显着改善相应分子的生物学特性。结果,芳族化合物的二氟烷基化已成为调节有机分子生物活性的有效策略。但是,2012年之前,选择性合成二氟烷基化芳烃的有效策略非常有限。传统的合成方法在苛刻的反应条件或狭窄的底物范围内都受到困扰,这极大地限制了它们的广泛应用,特别是对于生物活性分子的后期二氟烷基化。为了克服这些局限性,直接访问这些有价值的二氟烷基化骨架的方法是直接引入二氟烷基化基团(CF 2R)通过过渡金属催化的交叉偶联作用到芳环上。但是,由于某些易于被质子化,二聚化和/或生成其他未知副产物的二氟烷基化金属种类的不稳定性,难以选择性地控制催化循环以抑制这些副反应。在这种情况下,我们提出使用低成本和广泛使用的二氟烷基卤化物作为氟烷基源,用于通过交叉偶联进行过渡金属催化的二氟烷基化反应。









































 京公网安备 11010802027423号
京公网安备 11010802027423号