当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dissolution of CuS Particles with Fe(III) in Acidic Sulfate Solutions
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2018-09-05 , DOI: 10.1021/acs.iecr.8b01598 Wouter N. Wermink 1 , Geert F. Versteeg 2
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2018-09-05 , DOI: 10.1021/acs.iecr.8b01598 Wouter N. Wermink 1 , Geert F. Versteeg 2
Affiliation
|
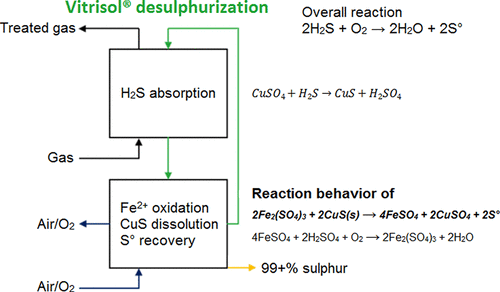
The dissolution of CuS particles with Fe3+ in acidic sulfate solutions was investigated in an oxygen-free environment. CuS particles, used in this study, were obtained from H2S removal operations from biogas with an acidic CuSO4 solution in a Vitrisol pilot absorber. The CuS particles were porous with an average diameter of 5.8 × 10–6 m. Zero orders of reaction for both Fe3+ and H2SO4 were determined. Full conversion of CuS could be obtained independent of temperature. The activation energy was determined to be EA = 22.0 kJ/mol. It seems plausible that the CuS dissolution reaction was affected by diffusion.
中文翻译:

Fe(III)在酸性硫酸盐溶液中溶解CuS颗粒
在无氧环境下,研究了Fe 3+对CuS颗粒在酸性硫酸盐溶液中的溶解作用。本研究中使用的CuS颗粒是通过在Vitrisol中试吸收塔中用酸性CuSO 4溶液从沼气中进行H 2 S去除操作而获得的。CuS颗粒是多孔的,平均直径为5.8×10 – 6 m。对于Fe 3+和H 2 SO 4,确定了零级反应。可以独立于温度获得CuS的完全转化。确定了激活能为Ê甲= 22.0千焦/摩尔。CuS溶解反应受扩散影响似乎是合理的。
更新日期:2018-09-05
中文翻译:

Fe(III)在酸性硫酸盐溶液中溶解CuS颗粒
在无氧环境下,研究了Fe 3+对CuS颗粒在酸性硫酸盐溶液中的溶解作用。本研究中使用的CuS颗粒是通过在Vitrisol中试吸收塔中用酸性CuSO 4溶液从沼气中进行H 2 S去除操作而获得的。CuS颗粒是多孔的,平均直径为5.8×10 – 6 m。对于Fe 3+和H 2 SO 4,确定了零级反应。可以独立于温度获得CuS的完全转化。确定了激活能为Ê甲= 22.0千焦/摩尔。CuS溶解反应受扩散影响似乎是合理的。










































 京公网安备 11010802027423号
京公网安备 11010802027423号