Journal of the Taiwan Institute of Chemical Engineers ( IF 5.5 ) Pub Date : 2018-08-21 , DOI: 10.1016/j.jtice.2018.07.045 Quoc-Thai Pham , Zong-Han Yao , Ya-Ting Chang , Fu-Ming Wang , Chorng-Shyan Chern
|
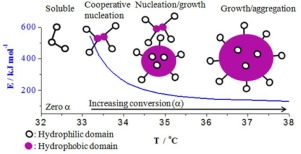
LCST phase transition kinetics of aqueous poly(N-isopropylacrylamide) (PNIPAM) solutions was studied by using non-isothermal differential scanning calorimeter (DSC) and isothermal dynamic light scattering (DLS) techniques. The DSC data obtained from 5 and 10 wt% PNIPAM solutions were used in isoconversional kinetics analysis. The resultant large activation energy (Eα) and pre-exponential factor indicated that the phase transition occurred by cooperatively breaking multiple hydrogen bonds. Eα decreased with increasing temperature approximately in a hyperbola form. Theoretical nucleation and growth models were used to adequately describe the temperature dependent Eα. Furthermore, the phase transition process obeyed Avrami–Erofeev nucleation and growth models. As to the 0.05 wt% PNIPAM solution using the isothermal DLS technique, the reaction rate constant was determined at different temperatures, and the Avrami–Arofeev nucleation and growth models adopted to predict the phase transition process.
中文翻译:

聚(N-异丙基丙烯酰胺)水溶液的LCST相变动力学
通过使用非等温差示扫描量热仪(DSC)和等温动态光散射(DLS)技术研究了聚(N-异丙基丙烯酰胺)(PNIPAM)水溶液的LCST相变动力学。从5重量%和10重量%的PNIPAM溶液获得的DSC数据用于等转化动力学分析。将得到的大的活化能(Ë α)和预指数因子表示的相变发生由断裂协同多个氢键。È α与双曲线形式大致随着温度的升高减少。理论成核和生长模型来充分地描述依赖于温度的È α。此外,相变过程遵循了Avrami-Erofeev的成核和生长模型。对于使用等温DLS技术的0.05 wt%PNIPAM溶液,在不同温度下确定反应速率常数,并采用Avrami-Arofeev成核和生长模型预测相变过程。











































 京公网安备 11010802027423号
京公网安备 11010802027423号