当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Why nature chose the Mn4CaO5 cluster as water-splitting catalyst in photosystem II: a new hypothesis for the mechanism of O–O bond formation
Dalton Transactions ( IF 3.5 ) Pub Date : 2018-08-21 00:00:00 , DOI: 10.1039/c8dt01931b Biaobiao Zhang 1, 2, 3, 4 , Licheng Sun 1, 2, 3, 4, 5
Dalton Transactions ( IF 3.5 ) Pub Date : 2018-08-21 00:00:00 , DOI: 10.1039/c8dt01931b Biaobiao Zhang 1, 2, 3, 4 , Licheng Sun 1, 2, 3, 4, 5
Affiliation
|
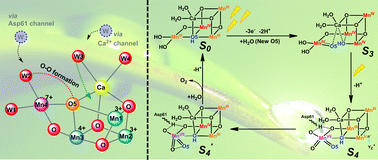
Resolving the questions, namely, the selection of Mn by nature to build the oxygen-evolving complex (OEC) and the presence of a cubic Mn3CaO4 structure in OEC coupled with an additional dangling Mn (Mn4) via μ-O atom are not only important to uncover the secret of water oxidation in nature, but also essential to achieve a blueprint for developing advanced water-oxidation catalysts for artificial photosynthesis. Based on the important experimental results reported so far in the literature and on our own findings, we propose a new hypothesis for the water oxidation mechanism in OEC. In this new hypothesis, we propose for the first time, a complete catalytic cycle involving a charge-rearrangement-induced MnVII–dioxo species on the dangling Mn4 during the S3 → S4 transition. Moreover, the O–O bond is formed within this MnVII–dioxo site, which is totally different from that discussed in other existing proposals.
中文翻译:

大自然为何在光系统II中选择Mn 4 CaO 5团簇作为水分解催化剂:O-O键形成机理的新假设
解决这些问题的方法是,通过自然选择锰来构建析氧络合物(OEC),以及在OEC中存在立方Mn 3 CaO 4结构,并通过μ-O原子与另外的悬空Mn(Mn4)耦合。不仅对于揭示自然界中水氧化的秘密非常重要,而且对于实现开发用于人工光合作用的高级水氧化催化剂的蓝图至关重要。根据迄今为止在文献中报道的重要实验结果和我们自己的发现,我们提出了OEC中水氧化机理的新假设。在这个新的假设中,我们首次提出了一个完整的催化循环,该循环涉及电荷重排诱导的Mn VII。在S 3 →S 4过渡期间,悬空的Mn4上的–dioxo物种。此外,O-O键在该Mn VII- dioxo位点内形成,这与其他现有提案中讨论的完全不同。
更新日期:2018-08-21
中文翻译:

大自然为何在光系统II中选择Mn 4 CaO 5团簇作为水分解催化剂:O-O键形成机理的新假设
解决这些问题的方法是,通过自然选择锰来构建析氧络合物(OEC),以及在OEC中存在立方Mn 3 CaO 4结构,并通过μ-O原子与另外的悬空Mn(Mn4)耦合。不仅对于揭示自然界中水氧化的秘密非常重要,而且对于实现开发用于人工光合作用的高级水氧化催化剂的蓝图至关重要。根据迄今为止在文献中报道的重要实验结果和我们自己的发现,我们提出了OEC中水氧化机理的新假设。在这个新的假设中,我们首次提出了一个完整的催化循环,该循环涉及电荷重排诱导的Mn VII。在S 3 →S 4过渡期间,悬空的Mn4上的–dioxo物种。此外,O-O键在该Mn VII- dioxo位点内形成,这与其他现有提案中讨论的完全不同。









































 京公网安备 11010802027423号
京公网安备 11010802027423号