当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Triple Mode of Alkylation with Ethyl Bromodifluoroacetate: N, or O‐Difluoromethylation, N‐Ethylation and S‐(ethoxycarbonyl)difluoromethylation
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2018-09-04 , DOI: 10.1002/adsc.201800824 Arghya Polley 1, 2 , Gurupada Bairy 1, 2 , Pritha Das 1 , Ranjan Jana 1, 2
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2018-09-04 , DOI: 10.1002/adsc.201800824 Arghya Polley 1, 2 , Gurupada Bairy 1, 2 , Pritha Das 1 , Ranjan Jana 1, 2
Affiliation
|
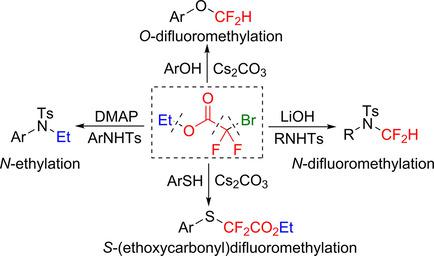
In this report, we have explored a triple mode of chemical reactivity of ethyl bromodifluoroacetate. Typically, bromodifluoroacetic acid has been used as a difluorocarbene precursor for difluoromethylation of soft nucleophiles. Here we have disclosed nucleophilicity and base dependent divergent chemical reactivity of ethyl bromodifluoroacetate. It furnishes lithium hydroxide and cesium carbonate promoted difluoromethylation of tosyl‐protected aniline and electron‐deficient phenols respectively. Interestingly, switching the base from lithium hydroxide to 4‐N,N‐dimethylamino pyridine (DMAP) tosyl‐protected anilines afforded the corresponding N‐ethylation product. Whereas, highly nucleophilic thiophenols furnished the corresponding S‐carboethoxydifluoromethylation product via a rapid SN2 attack to the bromine atom prior to the ester hydrolysis. This mechanistic divergence was established through several control experiments. It was revealed that difluoromethylation reaction proceeds through a tandem in situ ester hydrolysis/decarboxylative‐debrominative difluorocarbene formation and subsequent trapping by the soft nucleophile‐NHTs or electron‐deficient phenolic −OH groups. In the presence of DMAP the hydrolysis of the ester is perturbed instead a nucleophilic attack at the ethyl moiety provides the N‐ethylation product. Hence, besides the development of a practical base‐promoted N‐difluoromethylation of amines and electron‐deficient phenols, divergent reactivity pattern of inexpensive and user‐friendly ethyl bromodifluoroacetate has been explored.
中文翻译:

溴二氟乙酸乙酯合成烷基的三重模式:N或O-二氟甲基化,N-乙基化和S-(乙氧羰基)二氟甲基化
在这份报告中,我们探索了溴二氟乙酸乙酯的化学反应的三重模式。通常,溴二氟乙酸已被用作软亲核试剂的二氟甲基化的二氟卡宾前体。在这里,我们已经公开了溴二氟乙酸乙酯的亲核性和碱依赖性发散化学反应性。它提供了氢氧化锂和碳酸铯,分别促进了甲苯磺酰基保护的苯胺和缺电子的酚的二氟甲基化作用。有趣的是,将碱从氢氧化锂转变为4- N,N-二甲基氨基吡啶(DMAP)甲苯磺酰基保护的苯胺,得到了相应的N-乙基化产物。而高度亲核的苯硫酚提供了相应的S羰基乙氧基二氟甲基化产物在酯水解之前通过快速的S N 2攻击溴原子而形成。这种机制差异是通过几个控制实验确定的。结果表明,二氟甲基化反应是通过串联原位酯水解/脱羧-脱溴化二氟卡宾的形成并随后被软亲核试剂-NHTs或电子缺陷型酚-OH基团捕获而进行的。在DMAP的存在下,酯的水解受到干扰,相反,在乙基部分的亲核攻击提供了N-乙基化产物。因此,除了开发实用的碱促氮素胺和电子不足的酚的二氟甲基化,已开发出廉价且用户友好的溴二氟乙酸乙酯的不同反应模式。
更新日期:2018-09-04
中文翻译:

溴二氟乙酸乙酯合成烷基的三重模式:N或O-二氟甲基化,N-乙基化和S-(乙氧羰基)二氟甲基化
在这份报告中,我们探索了溴二氟乙酸乙酯的化学反应的三重模式。通常,溴二氟乙酸已被用作软亲核试剂的二氟甲基化的二氟卡宾前体。在这里,我们已经公开了溴二氟乙酸乙酯的亲核性和碱依赖性发散化学反应性。它提供了氢氧化锂和碳酸铯,分别促进了甲苯磺酰基保护的苯胺和缺电子的酚的二氟甲基化作用。有趣的是,将碱从氢氧化锂转变为4- N,N-二甲基氨基吡啶(DMAP)甲苯磺酰基保护的苯胺,得到了相应的N-乙基化产物。而高度亲核的苯硫酚提供了相应的S羰基乙氧基二氟甲基化产物在酯水解之前通过快速的S N 2攻击溴原子而形成。这种机制差异是通过几个控制实验确定的。结果表明,二氟甲基化反应是通过串联原位酯水解/脱羧-脱溴化二氟卡宾的形成并随后被软亲核试剂-NHTs或电子缺陷型酚-OH基团捕获而进行的。在DMAP的存在下,酯的水解受到干扰,相反,在乙基部分的亲核攻击提供了N-乙基化产物。因此,除了开发实用的碱促氮素胺和电子不足的酚的二氟甲基化,已开发出廉价且用户友好的溴二氟乙酸乙酯的不同反应模式。











































 京公网安备 11010802027423号
京公网安备 11010802027423号