当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iodine‐Promoted One‐pot Synthesis of Highly Substituted 4‐Aminopyrroles and Bis‐4‐aminopyrrole from Aryl Methyl Ketones, Arylamines, and Enamines
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2018-09-13 , DOI: 10.1002/adsc.201800899 Hitesh B. Jalani 1 , Jyotirling R. Mali 2 , Hyejun Park 1 , Jae Kyun Lee 3 , Kiho Lee 4 , Kyeong Lee 2 , Yongseok Choi 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2018-09-13 , DOI: 10.1002/adsc.201800899 Hitesh B. Jalani 1 , Jyotirling R. Mali 2 , Hyejun Park 1 , Jae Kyun Lee 3 , Kiho Lee 4 , Kyeong Lee 2 , Yongseok Choi 1
Affiliation
|
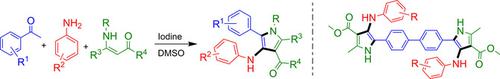
An iodine‐promoted one‐pot synthesis of functionally diverse and highly substituted 4‐aminopyrroles directly from aryl methyl ketones, arylamines, and enamines was developed. The reaction involves in‐situ oxidation of aryl methyl ketone to glyoxal, subsequent imine formation by aniline, followed by nucleophilic addition of enamine, and cyclization to afford highly substituted 4‐aminopyrroles. This reaction involved the formation of two C−N bonds and one C−C bond by a formal [1+1+3] annulation approach. The present method provides an interesting framework of two 4‐aminopyrrole units directly attached to a biphenyl core by the reaction of 4,4′‐diacyl biphenyl, amine, and enamine groups. This Hantzsch‐type one‐pot reaction provides diverse 4‐aminopyrroles, which could be useful in medicinal/material chemistry.
中文翻译:

由芳基甲基酮,芳胺和烯胺经碘促进的高取代度4-氨基吡咯和双4-氨基吡咯的一锅合成
直接由芳基甲基酮,芳基胺和烯胺开发了碘促进的一锅合成功能多样且高度取代的4-氨基吡咯。反应包括将芳基甲基酮原位氧化为乙二醛,随后通过苯胺形成亚胺,然后亲核加成烯胺,然后环化得到高度取代的4-氨基吡咯。该反应涉及通过正式的[1 + 1 + 3]环化方法形成两个C-N键和一个C-C键。本方法提供了一个有趣的框架,即通过4,4'-二酰基联苯,胺和烯胺基团的反应直接连接到联苯核上的两个4-氨基吡咯单元。这种Hantzsch型单锅反应可提供多种4-氨基吡咯,可用于药物/材料化学。
更新日期:2018-09-13
中文翻译:

由芳基甲基酮,芳胺和烯胺经碘促进的高取代度4-氨基吡咯和双4-氨基吡咯的一锅合成
直接由芳基甲基酮,芳基胺和烯胺开发了碘促进的一锅合成功能多样且高度取代的4-氨基吡咯。反应包括将芳基甲基酮原位氧化为乙二醛,随后通过苯胺形成亚胺,然后亲核加成烯胺,然后环化得到高度取代的4-氨基吡咯。该反应涉及通过正式的[1 + 1 + 3]环化方法形成两个C-N键和一个C-C键。本方法提供了一个有趣的框架,即通过4,4'-二酰基联苯,胺和烯胺基团的反应直接连接到联苯核上的两个4-氨基吡咯单元。这种Hantzsch型单锅反应可提供多种4-氨基吡咯,可用于药物/材料化学。











































 京公网安备 11010802027423号
京公网安备 11010802027423号