Journal of CO2 Utilization ( IF 7.2 ) Pub Date : 2018-08-18 , DOI: 10.1016/j.jcou.2018.07.018 Martin Alliati , Danhua Mei , Xin Tu
|
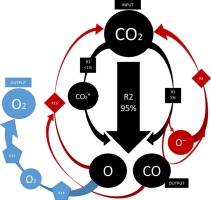
The conversion of CO2 into value-added chemicals or fuels has attracted much attention over the past years. Plasma technology represents a highly promising alternative due to its non-equilibrium nature, deemed crucial for CO2 dissociation reactions. Gaining a deep understanding of the reaction mechanisms involved under plasma conditions is essential to improve the performance of such processes. Among other theoretical calculations, plasma chemical kinetic modelling constitutes a very suitable approach to address this challenge. In this work, a zero-dimensional model of a dielectric barrier discharge (DBD) reactor is applied to CO2 splitting, providing a novel approach for including experimental parameters as discharge power and flow rate based on the analysis of the different scales involved. The model choices is extensively discussed as regards experimental parameters, cross-sectional data and the chemical reactions considered. The predictions of the model are in good agreement with existing experimental data and therefore the model is considered valid to analyse the CO2 splitting reaction mechanism based on its results. It is concluded that the electron impact dissociation is the dominant process towards CO2 conversion, which could explain the low energy efficiency achieved since only ∼10% of the electron energy is consumed by mechanism. The remaining energy would be lost towards vibrational excitation not leading to CO2 dissociation in DBD reactors.
中文翻译:

介质阻挡放电中CO 2的等离子体活化:从微放电到反应器规模的化学动力学模型
在过去的几年中,将CO 2转化为增值化学品或燃料备受关注。等离子体技术由于其非平衡性质而被认为是非常有前途的替代方法,这种性质被认为对CO 2离解反应至关重要。对等离子体条件下涉及的反应机理的深入了解对于改善此类过程的性能至关重要。在其他理论计算中,等离子体化学动力学建模是解决此难题的非常合适的方法。在这项工作中,电介质阻挡放电(DBD)反应器的零维模型应用于CO 2分离,提供了一种新颖的方法,可基于对所涉及的不同比例的分析,将实验参数作为放电功率和流速包括在内。关于实验参数,横截面数据和所考虑的化学反应,广泛讨论了模型的选择。该模型的预测与现有的实验数据非常吻合,因此该模型被认为可有效地根据其结果分析CO 2分解反应机理。结论是,电子冲击解离是产生CO 2的主要过程。转换,这可以解释由于仅约10%的电子能量被机制消耗而实现的低能量效率。剩余的能量将因振动激发而损失,而不会导致DBD反应器中的CO 2分解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号