Acta Biomaterialia ( IF 9.4 ) Pub Date : 2018-08-19 , DOI: 10.1016/j.actbio.2018.08.019 Soyoung Son , Veerasikku G. Deepagan , Sol Shin , Hyewon Ko , Jiwoong Min , Wooram Um , Jueun Jeon , Seunglee Kwon , Eun Sook Lee , Minah Suh , Doo Sung Lee , Jae Hyung Park
|
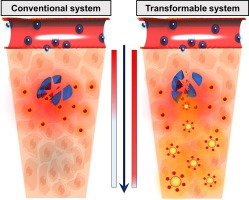
Since delivering drugs to an entire tumoral region leads to high therapeutic efficacy and good prognosis, achieving deep tumoral penetration of drugs is a major issue in cancer treatment. In this regard, conventional nanomedicines (> 50 nm) have shown limitations in cancer therapy, primarily attributed to the heterogeneous distribution of drugs because of the physiological barrier of the tumor interstitial space. To address this issue, we prepared transformable hybrid nanoparticles (TNPs) consisting of a pH-responsive nanocarrier (PEG-PBAE) and doxorubicin (DOX)-conjugated ultrasmall (< 3 nm) gold nanoparticles (nanosatellites). It has been shown that PEG-PBAE can serve as a reservoir for nanosatellites and release them in mildly acidic conditions (pH 6.5), mimicking the tumor microenvironment. When DOX-loaded TNPs (DOX-TNPs) were intravenously injected into tumor-bearing mice, they successfully accumulated and dissociated at the extracellular level of the tumor, leading to the disclosure of nanosatellites and free DOX. While the free DOX accumulated in tumor tissue near blood vessels, the deeply diffused nanosatellites were taken up by the tumor cell, followed by the release of DOX via cleavage of pH-responsive ester linkages in the nanosatellites at the intracellular level. Consequently, the DOX-TNPs effectively suppressed tumor growth through improved tumor penetration of DOX, suggesting their promising potential as a cancer nanomedicine.
Statement of Significance
Deep tumor penetration of anticancer drug is an important issue for high therapeutic efficacy. If the drugs cannot reach cancer cells in a sufficient concentration, their effectiveness will be limited. In this regard, conventional nanomedicine showed only modest therapeutic efficacy since they cannot deliver their payloads to the deep site of tumor tissue. This heterogeneous distribution of the drug is primarily attributed to the physiological barriers of the tumor microenvironment, including a dense extracellular matrix. To surmount this challenge, we developed tumor acidity-triggered transformable nanoparticles. By encapsulating doxorubicin-conjugated ultrasmall gold nanosatellites into the nanoparticles, the drug was not significantly bound to genetic materials, resulting in its minimal sequestration near the vasculature and deep tumor penetration. Our strategy could resolve not only the poor penetration issue of the drug but also its restricted tumor accumulation, suggesting the potential as an effective nanotherapeutics.
中文翻译:

具有超小金纳米粒子的可转化杂化纳米粒子,可深入肿瘤
由于将药物递送到整个肿瘤区域导致高治疗功效和良好的预后,因此实现药物的深入肿瘤渗透是癌症治疗中的主要问题。在这方面,常规的纳米药物(> 50 nm)在癌症治疗中显示出局限性,这主要归因于肿瘤间隙空间的生理屏障,药物的异质分布。为解决此问题,我们制备了可转化的杂化纳米颗粒(TNP),该杂化纳米颗粒由pH响应纳米载体(PEG-PBAE)和阿霉素(DOX)缀合的超小(<3 nm)金纳米颗粒(纳米卫星)组成。研究表明,PEG-PBAE可以充当纳米卫星的储存库,并在弱酸性条件下(pH 6.5)释放它们,从而模仿了肿瘤的微环境。当将载有DOX的TNP(DOX-TNP)静脉注射到荷瘤小鼠中时,它们在肿瘤的细胞外水平成功积聚并解离,从而导致了纳米卫星和游离DOX的出现。当游离的DOX累积在血管附近的肿瘤组织中时,深度扩散的纳米卫星被肿瘤细胞吸收,随后通过在细胞内水平上裂解纳米卫星中的pH响应酯键来释放DOX。因此,DOX-TNP通过改善DOX的肿瘤渗透性而有效地抑制了肿瘤的生长,表明它们作为癌症纳米药物的潜力很大。当游离的DOX累积在血管附近的肿瘤组织中时,深度扩散的纳米卫星被肿瘤细胞吸收,随后通过在细胞内水平上裂解纳米卫星中的pH响应酯键来释放DOX。因此,DOX-TNP通过改善DOX的肿瘤渗透性而有效地抑制了肿瘤的生长,表明它们作为癌症纳米药物的潜力很大。当游离的DOX累积在血管附近的肿瘤组织中时,深度扩散的纳米卫星被肿瘤细胞吸收,随后通过在细胞内水平上裂解纳米卫星中的pH响应酯键来释放DOX。因此,DOX-TNP通过改善DOX的肿瘤渗透性而有效地抑制了肿瘤的生长,表明它们作为癌症纳米药物的潜力很大。
重要声明
抗癌药的深层肿瘤渗透是实现高疗效的重要问题。如果药物不能以足够的浓度到达癌细胞,则其有效性将受到限制。在这方面,常规的纳米药物仅显示适度的治疗功效,因为它们不能将其有效载荷递送至肿瘤组织的深处。药物的这种异质分布主要归因于肿瘤微环境的生理屏障,包括致密的细胞外基质。为了克服这一挑战,我们开发了肿瘤酸度触发的可转化纳米粒子。通过将结合阿霉素的超小型金纳米卫星包裹到纳米粒子中,该药物并未与遗传物质显着结合,导致其在脉管系统附近的隔离最少,并深入了肿瘤。我们的策略不仅可以解决药物渗透性差的问题,而且可以解决其局限性的肿瘤蓄积问题,这表明它是一种有效的纳米疗法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号