Acta Biomaterialia ( IF 9.4 ) Pub Date : 2018-08-19 , DOI: 10.1016/j.actbio.2018.08.018 Jing Xie , Demao Zhang , Chenchen Zhou , Quan Yuan , Ling Ye , Xuedong Zhou
|
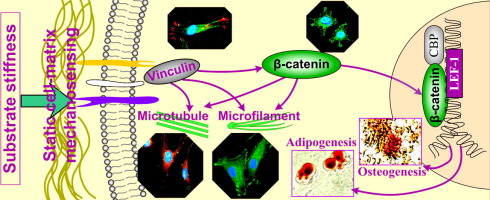
It is generally recognised that mesenchymal stem cells (MSCs) can differentiate into multiple lineages through guidance from the biophysical properties of the substrates. However, the precise biophysical mechanism that enables MSCs to respond to substrate properties remains unclear. In the current study, polydimethylsiloxane (PDMS) substrates with different stiffnesses were fabricated and the way in which the elastic modulus of the substrate regulated differentiation towards osteogenesis and adipogenesis in adipose-derived stromal cells (ASCs) was explored. Initially, a cell morphology change by SEM was observed between the stiff and soft substrates. The cytoskeleton stains including microfilament by F-actin and microtubule by α- and β-tubulin further showed a larger cell spreading area on the stiff substrate. Then the expression of vinculin, in charge for the linkage of adhesion molecules to the actin cytoskeleton, was enhanced on the stiff substrate. This change in focal adhesion plaque further triggered intracellular β-catenin signaling and promoted its nuclear translocation especially on the stiff substrate. The influence of β-catenin signaling on direct differentiation to osteogenic lineages was through direct binding between its downstream protein, Lef-1, and the osteogenic transcriptional factors, Runx2 and Osx, while on differentiation to adipogenic lineages was through modulating the expression of PPARγ. The imbalance of stiffness-induced β-catenin signaling finally induced a stronger osteogenesis and a weaker adipogenesis on the stiff substrate relative to those on the soft substrate. This study indicates the importance of stiffness on ASC differentiation and could help to increase understanding of the mechanism underlying molecular signal transduction from mechanosensing, mechanotransducing to stem cell differentiation.
Statement of Significance
Mesenchymal stem cells can differentiate into multiple lineages, such as adipogenesis, myogenesis, neurogenesis, angiogenesis and osteogenesis, through influence of biophysical properties of the extracellular matrix. However, the precise bio-mechanism that triggers stem cell differentiation in response to matrix biophysical properties remains unclear. In the current study, we provide a series of experiments involving the characterization of cell morphology, microfilament, microtubule and adhesion capacity of adipose-derived stromal cells (ASCs) in response to substrate stiffness, and further elucidation of cytoplasmic β-catenin-dependent signal transduction, nuclear translocation and resultant promoter activation of transcriptional factors for osteogenesis and adipogenesis. This study provides an explanation on deeper understanding of bio-mechanism underlying substrate stiffness-triggered β-catenin signal transduction from active mechanosensing, mechanotransducing to stem cell differentiation.
中文翻译:

底物弹性通过β-catenin转导调节脂肪来源的基质细胞向成骨和成脂的分化
通常认为,间充质干细胞(MSC)可以通过来自底物的生物物理特性的指导而分化成多个谱系。然而,尚不清楚使MSC能够响应底物特性的精确生物物理机制。在当前的研究中,制造了具有不同刚度的聚二甲基硅氧烷(PDMS)基底,并探索了基底的弹性模量调节脂肪来源的基质细胞(ASCs)向成骨和脂肪形成的分化的方式。最初,通过SEM观察到在硬质和软质基材之间的细胞形态变化。细胞骨架染色包括F-肌动蛋白的微丝和α-和β-微管蛋白的微管,进一步显示了在刚性底物上更大的细胞扩散区域。然后表达vinculin,负责粘附分子与肌动蛋白细胞骨架的连接的作用在刚性底物上得到增强。粘着斑斑的这种变化进一步触发了细胞内β-catenin信号传导,并促进了其核易位,尤其是在坚硬的基质上。β-catenin信号传导对成骨细胞系直接分化的影响是通过其下游蛋白Lef-1与成骨转录因子Runx2和Osx的直接结合而对成脂细胞系的分化是通过调节PPARγ的表达。刚度诱导的β-连环蛋白信号传导的失衡最终在硬质基底上引起了比软质基底上更强的成骨作用和较弱的脂肪生成。
重要声明
间充质干细胞可通过细胞外基质的生物物理特性影响而分化为多种谱系,例如脂肪生成,肌生成,神经生成,血管生成和成骨。但是,尚不清楚响应基质生物物理特性而触发干细胞分化的精确生物机制。在当前的研究中,我们提供了一系列实验,这些实验涉及表征脂肪基质细胞(ASC)对基质刚度的细胞形态,微丝,微管和粘附能力,并进一步阐明细胞质β-catenin依赖性信号骨形成和成脂的转录因子的转导,核易位和由此产生的启动子激活。











































 京公网安备 11010802027423号
京公网安备 11010802027423号